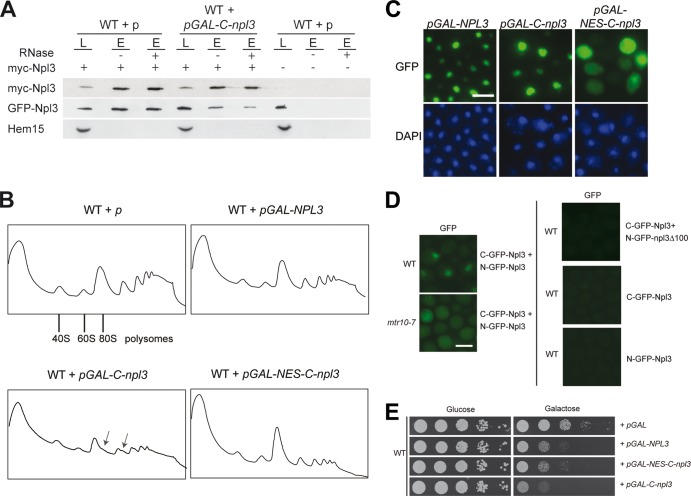
Fig 4.
Overexpression of the Npl3 dimerization domain is toxic and leads to halfmer formation. (A) Expression of the C-terminal domain of Npl3 inhibits the dimerization of the full-length proteins. Coimmunoprecipitations of myc-Npl3 and GFP-Npl3 were performed in the presence or absence of the C-terminal domain of Npl3. Western blot analyses, including the negative control protein Hem15, are shown. L, lysate; E, eluate. (B) Overexpression of the C-terminal domain of Npl3 disturbs proper monosome formation. Polysomal profiles of wild-type cells carrying the indicated plasmids are shown. Arrows indicate halfmers. (C) The C-terminal domain of Npl3 is localized to the nucleus. The localization of full-length Npl3 and the C-terminal domain of Npl3 tagged with GFP is shown with or without an NES in wild-type cells. Scale bars (C and D), 5 μm. (D) Npl3 dimerization occurs in the nucleus and in the cytoplasm. Split-GFP domains were used to detect the dimerized Npl3 proteins in vivo in the wild type and the mtr10-7 import receptor mutant. C-GFP-Npl3 and N-GFP-npl3Δ100 or the single (C- or N-GFP)-Npl3 proteins were used as negative controls. (E) Overexpression of the C-terminal domain of Npl3 in the nucleus severely inhibits cellular growth. Serial dilutions of wild-type strains carrying the indicated galactose-inducible vectors are shown on −URA glucose or galactose plates after incubation at 25°C for 3 days.