Abstract
Although such occurrences are rare, it should be recognized that certain vaccines might trigger serious neurological immune phenomena such as Guillain-Barre syndrome, seizures, cranial neuropathy, and acute disseminated encephalomyelitis (ADEM). Here we report on an elderly woman with ADEM following seasonal influenza vaccination who recovered after plasma exchange.
CASE REPORT
An 83-year-old Hispanic woman presented to the emergency department of Memorial Hermann Hospital—Texas Medical Center with 1 day of altered consciousness, weakness, and fever. During the previous 2 days, she had experienced retro-orbital pain and then progressed to decreased verbal response and inability to follow commands along with weakness and fever on the day of admission. Her past medical history was significant for hypertension, diabetes type 2, and previous ischemic stroke. Eight days prior, she had visited her primary care physician for an annual evaluation and received one single intramuscular dose of the 2012-to-2013 inactivated influenza vaccination (fluvirin; standard dose, 45 μg of hemagglutinin antigen). This was her only vaccine during the season.
On admission, she was found to be obtunded (Glasgow coma scale [GCS] score of 9), febrile (103.5°F), tachycardic (112 beats per min), tachypneic (32 breaths per min), and hypertensive (140/64 mm Hg) but with no hypoxemia. On neurological examination, she had quadriparesis, brisk reflexes, bilateral extensor plantar response, bilateral Hoffman's sign, and perioral fasciculations. A fundoscopic examination showed no evidence of papilledema, disc atrophy, neuritis, or hemorrhages. The results of the rest of the physical examination were within normal limits.
An initial computed tomography (CT) scan of the head was unremarkable. Cerebrospinal fluid (CSF) analysis revealed pleocytosis (44 cells/μl, 60% lymphocytes, 30% monocytes), protein of 136 g/dl, and glucose of 108 g/dl. Blood cell counts were normal. She had acute kidney injury with creatinine of 1.5 mg/dl and mild transaminitis (aspartate aminotransferase [AST], 98 mg/dl; alanine aminotransferase [ALT], 89 mg/dl). A presumptive diagnosis of infectious meningoencephalitis was made, and empirical treatment with ceftriaxone, ampicillin, vancomycin, and acyclovir was started. Despite 48 h of antibiotics, her mental status declined to a GCS score of 6 along with worsening focal findings and respiratory failure. She was emergently intubated and transferred to the intensive care unit for mechanical ventilation. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse abnormal fluid attenuated inversion recovery (FLAIR) signals in the periventricular white matter, splenium of the corpus callosum, bilateral thalami, bilateral mesial temporal lobes, midbrain, fornices, mammillary bodies, dorsal brain stem, and middle cerebellar peduncles as well as within the medulla oblongata (Fig. 1A). A 5-day course of intravenous methylprednisolone was given without clinical response. Further tests on the CSF, including bacterial and fungal cultures, PCR for herpes simplex virus (HSV), varicella-zoster virus (VZV), human herpesvirus 6 (HHV-6), enterovirus, JC and BK viruses, anti-NMDA (N-methyl-d-aspartate) receptor antibody and 14-3-3 protein, and flow cytometry for lymphoma, returned negative results. Serum studies for West Nile, St. Louis, Western equine, Eastern equine, and California encephalitis virus; Cryptococcus and Histoplasma antigens; and human immunodeficiency virus (HIV) viral load and a Quantiferon-TB test were all negative. Repeat MRI on day 12 showed progression and coalescence of T2 hyperintensity throughout the white matter of the corona radiata and centrum semiovale (Fig. 1B). After having ruled out infectious etiologies and due to the lack of clinical and radiological response, all antibiotics were discontinued on day 14 of admission.
Fig 1.
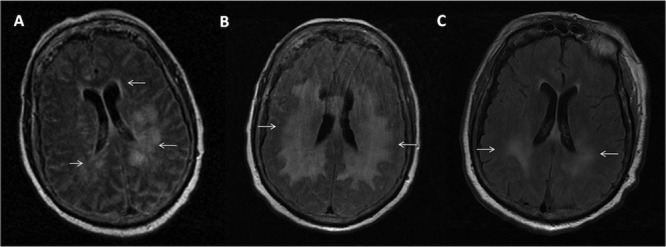
Progression of brain MRI changes in T2-weighted series. (A) Two days after admission, diffuse abnormal T2 hyperintensity signals, especially in periventricular white matter (arrows), were seen. (B) Progression and coalescence of T2 hyperintensity throughout periventricular white matter (arrows) after a course of steroids. (C) Significant decrease in T2 hyperintensity (arrows) after 5 sessions of plasma exchange.
The patient's clinical presentation—fever, altered consciousness, and multifocal neurologic deficits—combined with MRI findings that demonstrated extensive white matter lesions suggested the diagnosis of acute disseminated encephalomyelitis (ADEM). Therefore, plasma exchange was started on day 24, with 5 exchange sessions performed over 10 days. Dramatic clinical improvement was seen, and follow-up brain MRI undertaken at the end of therapy showed a significant decrease in T2 hyperintensity throughout the white matter (Fig. 1C). On day 42, the patient was discharged to a rehabilitation facility, with preserved alertness, motor function, speech, and spontaneous breathing. After 3 months, the patient developed pneumonia with septic shock and died. There was no relapse of neurological symptoms in this time interval. This confirmed our suspicion of ADEM, as the patient met level 1 criteria for its diagnosis by the presence of multifocal neurologic findings, MRI displaying diffuse white matter lesions, and the monophasic course of the disease (no relapse at 3 months). A final diagnosis of postvaccination ADEM was made after exclusion of infectious and noninfectious etiologies along with the recent exposure to influenza vaccination 8 days prior to admission.
ADEM is an inflammatory demyelinating disease of the central nervous system with an estimated annual incidence of 0.8 per 100,000 (1). Infectious causes are the most frequent etiology, and postvaccination ADEM accounts for less than 5% of all the ADEM cases (2). It has been associated with several vaccines, such as rabies, diphtheria-tetanus-polio (dPT), influenza, smallpox, measles, mumps, rubella, Japanese B encephalitis, pertussis, and the hepatitis B vaccine (3). The incidence rate is as low as 0.1 to 0.2 per 100,000 vaccinated individuals (1). A literature review identified 15 published reports of ADEM following influenza vaccination since 1982 (4). It has been noted to occur more frequently after primary vaccination than after revaccination, and the majority of the cases have been described in patients who received inactivated influenza vaccine (5).
Clinical presentation includes fever, altered consciousness, and multifocal neurological findings, which typically appear within 1 day to 3 weeks of immunization and show rapid progression. Focal findings depend on the location and degree of demyelination (3). MRI is a valuable tool for diagnosis, revealing multifocal or extensive white matter or deep gray matter lesions (thalamus and basal ganglia) within 5 to 14 days of symptom onset (2).
However, it should be remembered that ADEM or any other adverse event that occurs subsequent to a vaccine administration may not necessarily be attributable to the vaccine and might merely be a temporal association (6). This distinction between temporal coincidence and causality is often challenging due to the lack of a specific diagnostic test; thus, certain causality criteria have been developed. A recent Clinical Immunization Safety Assessment (CISA) network review of 212 Vaccine Adverse Event Reporting System (VAERS) reports of nonfatal serious neurological events following H1N1 vaccination showed that ADEM comprised 3.8% (8/212) of the diagnoses (5). Utilizing the modified WHO criteria for causality assessment, the diagnosis of postvaccination ADEM was deemed “possible” in only 50% (4/8) of the cases assessed, with none being classified as “probable” or “definite.” Hence, the use of causality criteria is essential prior to making a diagnosis of postvaccination ADEM. In our patient, the clinical event showed a plausible time relationship with the vaccine administration and, in the absence of other possible etiology, the diagnosis of postvaccination ADEM was “very likely/certain” (7).
Most treatment options for ADEM are based on empirical and observational evidence. Early institution of therapy with daily methylprednisolone for 3 to 5 days followed by a month-long prednisone taper is generally accepted as the first-line therapy. For steroid-unresponsive patients, such as ours, plasma exchange is recommended. The usual course involves 7 exchange sessions conducted over 2 weeks, with improvements frequently seen after the first plasma exchange (2). Intravenous immunoglobulin (IVIG) is reserved for patients with ADEM who fail to respond to corticosteroid treatment and/or when plasma exchange is contraindicated or not available. With timely treatment, full recovery is seen in 50% to 75% of the patients, usually within 1 to 6 months (2). Once patients have recovered, it is advisable to avoid immunization in the 6-month follow-up period, since relapses may occur following routine vaccinations (2).
In summary, this case illustrates a rare case of post-influenza vaccination acute disseminated encephalomyelitis, emphasizing the paramount importance of recognizing recent vaccination exposures. Clinicians must be aware of the possible adverse neurologic events following immunization and should consider postvaccination ADEM in patients with altered consciousness and multifocal neurological findings after recent vaccination, as early recognition is essential for full recovery.
ACKNOWLEDGMENTS
J.D.M. and B.B.-R. conceived the study, participated in its design and coordination, and drafted the manuscript. J.D.M., B.B.-R., G.D., and B.J.M. wrote and revised the manuscript. J.D.M. and B.J.M. were the physicians responsible for the patient.
We declare that we have no conflicts of interest.
We received no funding support for this work.
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.Menge T, Kieseier BC, Nessler S, Hemmer B, Hartung HP, Stuve O. 2007. Acute disseminated encephalomyelitis: an acute hit against the brain. Curr. Opin. Neurol. 20:247–254 [DOI] [PubMed] [Google Scholar]
- 2.Bennetto L, Scolding N. 2004. Inflammatory/post-infectious encephalomyelitis. J. Neurol. Neurosurg. Psychiatry 75(Suppl 1):i22–i28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. 2008. Post-vaccination encephalomyelitis: literature review and illustrative case. J. Clin. Neurosci. 15:1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoamanesh A, Traboulsee A. 2011. Acute disseminated encephalomyelitis following influenza vaccination. Vaccine 29:8182–8185 [DOI] [PubMed] [Google Scholar]
- 5.Williams SE, Pahud BA, Vellozzi C, Donofrio PD, Dekker CL, Halsey N, Klein NP, Baxter RP, Marchant CD, Larussa PS, Barnett ED, Tokars JI, McGeeney BE, Sparks RC, Aukes LL, Jakob K, Coronel S, Sejvar JJ, Slade BA, Edwards KM. 2011. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine 29:8302–8308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sejvar JJ, Kohl KS, Bilynsky R, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, Gigugu J, Katikanemi L, Khyri-Bulos N, Oleske J, Tapiainen T, Wiznitzer M. 2007. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 25:5771–5792 [DOI] [PubMed] [Google Scholar]
- 7.Collet JP, MacDonald N, Cashman N, Pless R. 2000. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Bull. World Health Organ. 78:178–185 [PMC free article] [PubMed] [Google Scholar]


