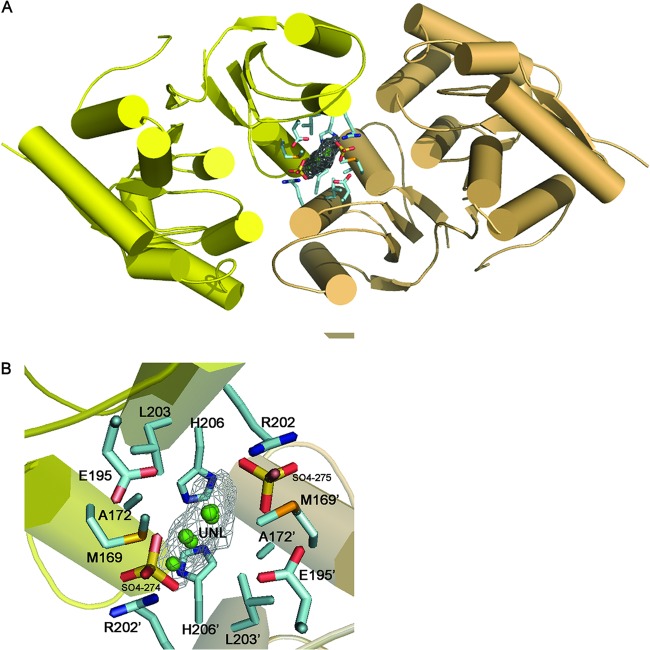
Fig 6.
Potential site for modulating protein-protein interaction. An unidentified ligand (UNL) at the NaLdcA dimer interface (monomers are shown in yellow and beige) reveals a novel binding site that could be targeted for modulating oligomerization and activity of LdcA enzymes. A close-up of this site shows the residues that were found to interact with the ligand. Glu195 is highly conserved in other homologs as displayed in Fig. 3. Arg202 and His206 are also conserved as displayed in Fig. 5. A combination of highly conserved and less-conserved residues at this site suggests that it could be explored for the design of a general LdcA dimer inhibitor whose properties could be modified for individual LdcA proteins.