Abstract
An epidemic of human H7N9 influenza virus infection recently emerged in China whose clinical features include high mortality and which has also resulted in serious economic loss. The novel reassortant avian-origin influenza A (H7N9) virus which was the causative agent of this epidemic raised the possibility of triggering a large-scale influenza pandemic worldwide. It seemed likely that fast molecular detection assays specific for this virus would be in great demand. Here, we report a one-step reverse transcription–loop-mediated isothermal amplification (RT-LAMP) method for rapid detection of the hemagglutinin (HA) and neuraminidase (NA) genes of H7N9 virus, the minimum detection limit of which was evaluated using in vitro RNA transcription templates. In total, 135 samples from clinical specimens (from either patients or poultry) were tested using this method in comparison with the real-time PCR recommended by the World Health Organization (WHO). Our results showed that (i) RT-LAMP-based trials can be completed in approximately 12 to 23 min and (ii) the detection limit for the H7 gene is around 10 copies per reaction, similar to that of the real-time PCR, whereas the detection limit for its counterpart the N9 gene is 5 copies per reaction, a 100-fold-higher sensitivity than the WHO-recommended method. Indeed, this excellent performance of our method was also validated by the results for a series of clinical specimens. Therefore, we believe that the simple, fast, and sensitive method of RT-LAMP might be widely applied for detection of H7N9 infections and may play a role in prevention of an influenza pandemic.
INTRODUCTION
A serious epidemic of human infections with a novel avian influenza A (H7N9) virus has emerged in China, the first case of which was recorded in eastern China in February 2013 (1). Patients with this viral infection exhibited influenzalike illness and severe pneumonia (2). As of 31 May 2013, it has already resulted in no less than 131 laboratory-confirmed cases, with 39 deaths. Economic losses due to this novel avian reassortant influenza A virus have been pegged at more than $6.5 billion, and it still poses a serious threat to public health (3). Of particular note, the virus is still evolving to acquire strategies for effective human-to-human transmission and could have the possibility to initiate the next pandemic (4, 5).
For effective global monitoring and control of the H7N9 epidemic, it is necessary that the characterization of influenzalike cases, especially severe pneumonia in humans, and H7N9 avian influenza virus (AIV) detection in migratory birds and poultry be stringent (6). Currently, the sensitivity of the immunological colloidal-gold test method is quite low (7), making it difficult to detect H7N9 in samples from clinical cases. Quantitative real-time reverse transcription-PCR (rRT-PCR) of the H7N9 avian influenza virus hemagglutinin (HA) and neuraminidase (NA) genes (8, 9, 10), while being both sensitive and specific, requires expensive equipment, professional technology, and a complicated methodology which is not suitable for on-site detection or laboratories with poor conditions, especially in developing countries. There is, therefore, an urgent need to establish a simple, rapid, and sensitive method for the detection of the new H7N9 influenza virus.
Loop-mediated isothermal amplification (LAMP) is a novel nucleic acid amplification method with only the requirement of a temperature-controlled water bath (11). Given the rapidity, sensitivity, and efficiency of the strategy, it has been modified for application to a series of viral detections, such as H5N1 influenza virus (12, 13). In recent years, improvements in the LAMP method, such as visual detection of amplified product by adding calcein (14) or hydroxynaphthol blue (HNB) (15), which still require no other equipment and avoid aerosolized nucleic acid pollution, have made it easier to use in field testing or in resource-limited settings (16, 17). Very recently, Ge and coworkers developed an RT-LAMP assay integrated with a lateral flow device (RT-LAMP-LFD), which was successfully applied in the detection of H7N9 in hospital clinical specimens and also validated as a fast and convenient method (18). However, this method has limitations in that (i) the LFD device used is a patent-protected product (Bioustar), (ii) double-labeled primers are required for detection, and (iii) the cost is relatively high (19).
In this study, we developed a principal RT-LAMP method with HNB dye to detect the H7N9 avian influenza virus, which was not only validated with an array of clinical specimens (in total, 135 samples either from patients or different kinds of poultry) but also compared with real-time fluorescent quantitative PCR detection, the World Health Organization (WHO)-recommended method (of note, it is the most widely used method for detection of H7N9 avian influenza virus in laboratories).
MATERIALS AND METHODS
Strains and clinical specimens.
The H7N9 avian influenza virus strain (A/Nanjing/1/2013) was isolated from bronchoalveolar lavage fluid specimens obtained from the first diagnosed patients, in Nanjing City. Three subtypes of AIV (H7N1, H7N3, and H7N7) were kind gifts from Yuwei Gao (The Military Veterinary Institute, Changchun City, China). The other 11 virus samples (including H5N1 virus, H9N2 virus, H1N1 virus strains [2009], H3N2 virus, two species of human coronaviruses [HCoV-229E and HCoV-OC43], rhinovirus [N36], seasonal H1N1 influenza virus, adenovirus type 4, influenza B virus, and parainfluenza virus type 3) were kind gifts from Tao Jiang of the Military Medical Science Academy of the PLA, Beijing.
For clinical samples, 6 throat swab samples and 4 lower respiratory tract lavage fluid samples were collected beginning in April 2013 from 10 people infected with the H7N9 avian influenza virus in the Jiangsu province (samples are coded, completely anonymous, and approved by the local ethical board), 90 cloacal swabs and 25 throat swabs were collected on 3 April 2013 from chickens, ducks, and pigeons in the Nanjing Zijin Mountain live-poultry markets, and 10 cloacal swabs, serving as controls, were collected from specific-pathogen-free (SPF) chickens (21 days old).
Viral RNA isolation.
In a biosafety level 3 (BSL-3) laboratory or an enhanced level 2 laboratory, viral genomic RNA was extracted using the QIAamp RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and dissolved in a final volume of 50 μl of the eluent. To this, 1 μl (40 U) of an RNA enzyme inhibitor (TaKaRa, Dalian, China) was added, and the mixture placed at −80°C immediately.
Primer design.
To design specific primers to amplify conserved regions of the HA and NA genes of several subtypes, gene sequences of the A/Nanjing/1/2013 strain and H1, H3, H5, H7, and H9 subtype influenza viruses, as well as N1, N2, N7, and N9 subtype influenza viruses, respectively, were first obtained from the GISAID database (http://www.gisaid.org/). Subsequently, based on sequence alignments using ClustalX 2.11 (Des Higgins), followed by the use of Primer Explorer version 4 (http://primerexplorer.jp/elamp4.0.0/index.html), primers were obtained. Each set of primers included 2 outer primers, F3 and B3, two inner primers, FIP and BIP, and loop primers, LB or LF. Primers were obtained from Shanghai Invitrogen, with synthesis and purification by high-performance liquid chromatography (HPLC). After experimental verification, the preferred sequences were determined (Table 1).
Table 1.
Primers designed for the RT-LAMP assay for the H7N9 virus
| Assay and primera | Sequence (5′–3′) | Target gene, GenBank accession no. |
|---|---|---|
| H7-RT-LAMP | HA gene, KC896774.1 | |
| B3 | GAGCCATTTCATTTCTGCA | |
| F3 | TGAGAGGCGAGAAGGAAG | |
| BIP | ||
| B1c | TGACAAGGAAGCAATGGGATTC | |
| B2 | TCCTGATCTCCTACATGCA | |
| FIP | ||
| F1c | GCCTGATTCTCTGAGAATTTGCCT | |
| F2 | TGATGTCTGTTATCCTGGGA | |
| LB | TGGAATAAGAACTAATGGAGCAACC | |
| N9-RT-LAMP | NA gene, KC896776.1 | |
| B3 | ATAGCAGTCCCCTTCAGC | |
| F3 | GATGGGGCTAACACTTGG | |
| BIP | ||
| B1c | TAGATCAAAGCCCATTCAAGGTC | |
| B2 | GTCCATGAAAGATCCACTGTA | |
| FIP | ||
| F1c | TCAATGCATTTGGCACTTTTAACAT | |
| F2 | TAGGGAGGACAATAAGCAC | |
| LF | CGTATCCAGACCTCGAGGCT |
The FIP primers are long primers with two recognition sequences, F1c and F2, separated by hyphens; the BIP primers are also long primers with two recognition sequences, B1c and B2.
RT-LAMP reaction.
For the detection of the H7N9 avian influenza virus H7 and N9 genes by RT-LAMP (H7-RT-LAMP and N9-RT-LAMP, respectively), the total reaction mixture volume was 25 μl, consisting of 20 mmol/liter Tris-HCl (pH 8.8), 10 mmol/liter KCl, 10 mmol/liter (NH4)2SO4, 0.1% Triton X-100, 8 mmol/liter MgSO4, 0.8 mmol/liter betaine (Sigma, USA), 1.4 mmol/liter deoxynucleoside triphosphates (dNTPs) (Promega, USA), 8 U Bst DNA polymerase (New England BioLabs, USA), 10 U avian myeloblastosis virus (AMV) reverse transcriptase (New England BioLabs), primers (for FIP and BIP, 1.6 μM each, for F3 and B3, 0.2 μM each, and for LF and LB, 0.8 μM each), and 3 μl of the template.
Real-time scanning of turbidity at the desired temperature was achieved by using an LA-320C turbidimeter (Eiken Chemical Co. Ltd., Japan). The reaction was allowed to progress for 30 min at 63°C, and then the temperature was raised to 85°C for 2 min to stop the reaction. Turbidity values greater than 0.1 were considered positive. In addition to the turbidity meter, reaction outcomes could be observed visually in the RT-LAMP system based on a color change of blue for positive and violet for negative outcomes by using HNB (Sigma, USA). The reaction mixture was set up at a final concentration of 120 mM HNB and allowed to progress at 63°C for 30 min, using a common water bath or metal temperature bath, before observing the color change.
Real-time fluorescent quantitative PCR.
The method for detection of H7N9 avian influenza virus by real-time reverse transcription-PCR recommended by WHO (20) was utilized in strict accordance to their recommendations for reagents, procedure, and methodology and is referred to herein as H7-rRT-PCR and N9-rRT-PCR. H7 and N9 gene primers were synthesized by the Shanghai Invitrogen company, and the reactions were performed using the ABI 7500 fast real-time PCR system (Life Technologies, USA).
In vitro transcription.
Following the extraction of RNA from the A/Nanjing/1/2013 strain, fragments of the HA and NA genes were amplified by RT-PCR and TA cloned into the PCR II plasmid (Invitrogen, Inc., Carlsbad, CA). These were sequenced and named H7-PCR II and N9-PCR II. The H7-PCR and the N9-PCR plasmids were linearized using the restriction endonucleases SpeI and BamHI (TaKaRa, Dalian), respectively, and then used as templates for the RiboMax T7 in vitro transcription system (Promega, Madison, WI). After DNase treatment to eliminate template DNA, the RNA product was purified using an RNA purification kit (Tiangen, Beijing) and its concentration was determined spectrophotometrically using a Nanodrop 2000 (Thermo Scientific, USA).
Sensitivity and specificity of the assay.
The RNA products obtained from the in vitro transcription of the HA and NA genes were serially diluted to obtain a 10-fold gradient from 106 copies/μl to 1 copy/μl for HA and 5 × 105 copies/μl to 0.5 copies/μl for NA (note that 1 μl of the diluted transcript was added into the 25-μl-total-volume reaction mixture) to serve as a standard for testing the sensitivity of this assay and to establish detection limits for H7-RT-LAMP, H7-rRT-PCR, N9-RT-LAMP, and N9-rRT-PCR. All reactions were performed in triplicates.
To test specificity, the RNA extracts from the reference viruses causing influenzalike symptoms mentioned above (H7N9 virus, H5N1 virus, H9N2 virus, H1N1 virus [2009], H3N2 virus, adenovirus type 4, influenza B virus, and parainfluenza virus type 3) were subjected to H7-RT-LAMP and N9-RT-LAMP detection.
Clinical sample detection.
135 clinical specimens (Table 2) were assayed by RT-LAMP and real-time reverse transcription-PCR detection.
Table 2.
Comparison of RT-LAMP and rRT-PCR for detection of H7N9 AIV in 135 clinical samples
| Specimen type | Total no. of samples | No. of samples positive for indicated subtype using: |
|||
|---|---|---|---|---|---|
| H7-rRT-PCR | H7-RT-LAMP | N9-rRT-PCR | N9-RT-LAMP | ||
| Oral swabs from H7N9 patients | 6 | 6 | 6 | 5 | 6 |
| BALFa samples from H7N9 patients | 4 | 4 | 4 | 4 | 4 |
| Oral swabs from chickens | 25 | 3 | 3 | 0 | 3 |
| Cloacal scrapings from: | |||||
| Chickens | 58 | 15 | 15 | 4 | 14 |
| Ducks | 20 | 4 | 4 | 1 | 3 |
| Meat pigeons | 12 | 2 | 2 | 0 | 2 |
| SPF chickensb | 10 | 0 | 0 | 0 | 0 |
| Total | 135 | 34c | 34 | 14 | 32 |
BALF, bronchoalveolar lavage fluid.
Cloacal scrapings from SPF chickens served as the negative controls in the experiments.
These 34 positive sample were further confirmed by sequencing analyses.
RESULTS
Detection limit of the RT-LAMP method compared to that of real-time RT-PCR.
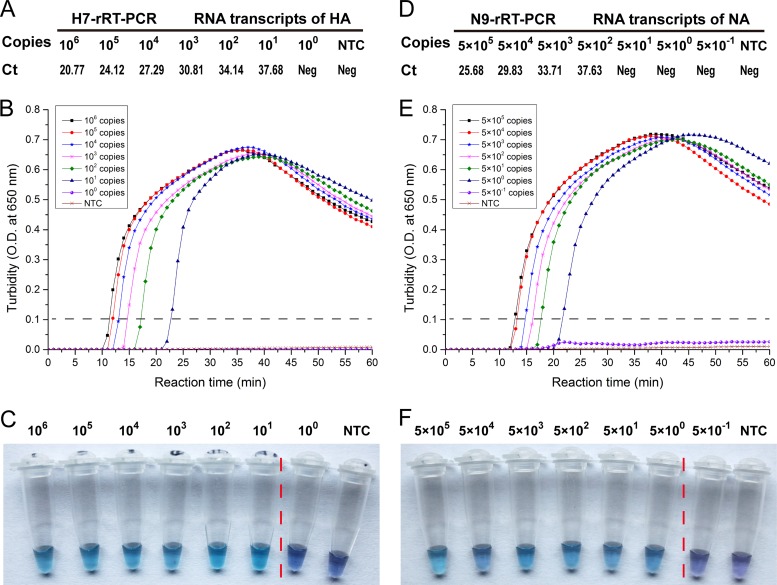
The sensitivity of the test was established by comparing the detection of serially diluted samples by both methods. The lower detection limit for both H7-RT-LAMP and H7-rRT-PCR was 101 copies per reaction mixture (Fig. 1B, C, and A). The N9-RT-LAMP detection limit was 5 × 100 copies per reaction mixture (Fig. 1E and F), while the detection limit was 5 × 102 copies per reaction mixture for N9-rRT-PCR (Fig. 1D). The sensitivity of N9-RT-LAMP is 100-fold greater than that of the real-time PCR.
Fig 1.
Limit of detection of H7N9 virus by RT-LAMP and rRT-PCR. The RNA products obtained from the in vitro transcription of the HA and NA genes were serially diluted to obtain 10-fold gradients from 106 copies/μl to 1 copy/μl for the HA gene and 5 × 105 copies/μl to 0.5 copies/μl for the NA gene for use as templates for amplification by rRT-PCR (A and D) and RT-LAMP (B, C, E, and F). Threshold cycle crossing values for rRT-PCR are indicated (Ct). RT-LAMP results were analyzed using an LA-320C turbidimeter (O.D., optical density) (B and E), as well as by color change using HNB (C and F). Two target genes were used in the assays; one is the HA gene (A to C) and the other is the NA gene (D to F).
H7-RT-LAMP and N9-RT-LAMP were completed the most quickly, taking 12 to 13 min to obtain positive results. At the minimum detection limit, results were available within 22 to 23 min (Fig. 1B and E). Direct visualization of the HNB dye-aided color change not only determined amplification but also did not require excess instruments, needing only a temperature-controlled bath, and the HNB dye results were consistent with the turbidity data.
Analysis of cross-reactivity of H7N9 AIV RT-LAMP.
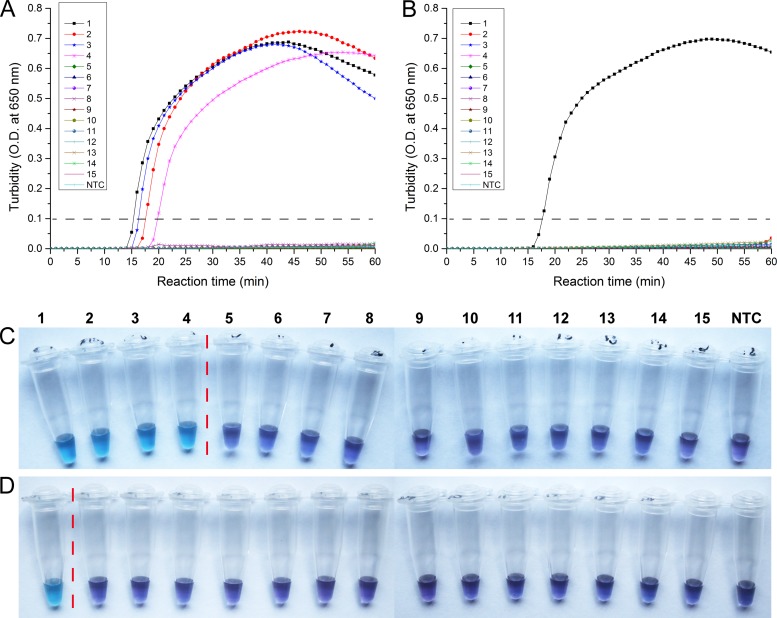
Using various types of viral genomic RNA sample preparations, we conducted RT-LAMP tests specific for either the HA gene or the NA gene. The results of H7-RT-LAMP were positive with H7N9, H7N1, H7N3, and H7N7 viruses (Fig. 2A and C), suggesting that it can be applied to the H7 subtype of avian influenza virus. With the exception of H7N9, the results of N9-RT-LAMP detection were negative with H7N1, H7N3, and H7N7 viruses (Fig. 2B and D), further indicating that this method has good discrimination of H7 subtype H7N9 avian influenza virus. As expected, other viral nucleic acid extracts (H5N1 and H9N2 AIV and other common respiratory viruses) did not give any positive results in either H7-RT-LAMP or N9-RT-LAMP, validating the high specificity of this method.
Fig 2.
Specificity of the RT-LAMP assay. Viral RNAs extracted from various control viruses and the reference virus were used. 1, H7N9 virus; 2, avian influenza A H7N1 virus; 3, avian influenza A H7N3 virus; 4, avian influenza A H7N7 virus; 5, avian influenza A H5N1 virus; 6, avian influenza A H9N2 virus; 7, human seasonal influenza A H3N2 virus; 8, 2009 swine-origin influenza virus A H1N1 virus; 9, human seasonal influenza A H1N1 virus; 10, HCoV-229E; 11, HCoV-OC43; 12, human rhinovirus N36; 13, influenza B virus; 14, adenovirus type 4; 15, parainfluenza virus type 3; NTC, no-template control. Detection by the HA-RT-LAMP (A) and NA-RT-LAMP (B) reactions was assessed by real-time turbidity assay using an LA-320c turbidimeter. Detection by the HA-RT-LAMP (C) and NA-RT-LAMP (D) reactions was also assessed by visible detection methods using hydroxynaphthol blue. The dashed gray lines in panels A and B and the red vertical dotted lines in panels C and D indicate the cutoffs for positive versus negative.
Evaluation of H7N9 AIV RT-LAMP assay using clinical and field samples.
To evaluate the sensitivity of RT-LAMP in clinical practice, 135 samples were subjected to both RT-LAMP and rRT-PCR detection simultaneously (Table 2). The sensitivities of both H7-RT-LAMP and H7-rRT-PCR were the same, with 34 of 135 samples detected as positive (confirmed by sequencing; the rest were negative). The sensitivity of NA-RT-LAMP was higher than that of NA-rRT-PCR, with 32 N9 RNA positives out of the 34 H7 nucleic acid-positive samples being detected by NA-RT-LAMP and only 14 of the 34 detected by NA-rRT-PCR. All of the above-described HA-positive but NA-negative specimens were confirmed to be due to infections of H7N9 virus by using viral cultivation combined with genomic sequencing. The positive coincidence rate of RT-LAMP was 94.12%, while that for rRT-PCR was only 41.18%.
DISCUSSION
We developed two sets of one-step RT-LAMP detection methods, one specific for the HA gene and the other for the NA gene. The sensitivities to the H7 gene by RT-LAMP and rRT-PCR (WHO recommended) were the same, and the sensitivity to the NA gene by RT-LAMP was 100 times greater than that of rRT-PCR (WHO recommended). Clinical application of the WHO-recommended method of N9-rRT-PCR in the China CDC laboratory has shown a high level of inconvenience and confusion due to a weak fluorescence signal detection rate. In addition, the use of HNB to observe a visible color change, using just an ordinary water bath, offers a detection sensitivity similar to that of the turbidity meter-based method. The major characteristics of the set of two RT-LAMP methods are that they are both rapid and specific, requiring only 11 to 23 min to determine the detection results and having no observed cross-reactivity to other respiratory viruses (H5N1, H9N2, H3N2, H1N1, etc.) that display infection symptoms similar to H7N9 infection scenarios.
Recently, Nie et al. also utilized the LAMP method for the detection of the H7N9 virus (21), but the detection time for the NA gene was relatively longer, about 38 to 58 min, and its sensitivity was low, i.e., similar to that of the WHO-recommended N9-rRT-PCR method for detection of the NA gene. This is still 100-fold lower than that of the RT-LAMP method we developed here, hence reducing its value for clinical application.
The high sensitivity of the RT-LAMP method in this study was confirmed by the detection of H7N9 in clinical samples. Ten confirmed cases were detected as positive using this method; poultry samples from a live-poultry market were detected with a positive rate of 20.87% (24/115), similar to a previous report (22). In the H7 DNA-positive samples, the rate of detection of the N9 gene by N9-RT-LAMP was significantly higher than that of the corresponding N9-rRT-PCR. The high-resolution detection of the N9 gene fragment can contribute to better differentiation from H7N1, H7N2, H7N3, H7N7, and other H7 virus subtypes (23). When the samples (especially poultry samples) are found to be H7 positive but N9 negative, they probably belong to H7N1, H7N2, H7N3, or H7N7 avian influenza viruses. Two alternative options are proposed here: (i) determination by both viral cultivation and viral genomic sequencing in a well-equipped laboratory and (ii) verification using the sensitive molecular method that is highly specific for only one out of the N1, N2, N3 and N7 genes (24). If the sample is found to be negative for H7 but positive for N9, the possibility of either H11N9 or a new recombinant virus could not be ruled out. That is why we strongly recommended that other approaches, especially those such as deep sequencing, be employed to address this further (25).
In summary, this study establishes a simple RT-LAMP method for fast detection of the H7N9 avian influenza virus, which was confirmed and evaluated using clinical specimens. The RT-LAMP method has the advantages of high sensitivity and accuracy and of not needing special equipment. For detection of the N9 subtype, the sensitivity was higher than that of the real-time fluorescent quantitative PCR method recommended by the World Health Organization (WHO). Therefore, we believe that the simple, fast, and sensitive method of RT-LAMP might be applied to the field detection of H7N9 infections (such as in poultry specimens and clinical patients) and could play a role in prevention of an influenza pandemic.
ACKNOWLEDGMENTS
This work was supported by the National S&T Project for Infectious Diseases Control (grants 2013ZX10004-103, 2013ZX10004-104, 2013ZX10004-203, 2013ZX10004-218, and 2012ZX10004801-004), the Shanghai Municipal Health and Family Planning Committee (grant 2013QLG009), National Natural Science Foundation of China (grant 31170124), Key Project of the Military Twelfth Five-Year Research Program of PLA (grants AWS11C001 and AWS11C009), Military Special Program of SMMU (grant 2012JS01), Key Technology R&D Program of Jiangsu Province, China (grants BE2012609 and BE2013603), Natural Science Foundation of Jiangsu Province, China (grants BK2011097 and BK2011098), Key Problems Project in Science and Technology of Nanjing Command, China (grants 10Z039 and 11Z040), and Key Project supported by the Medical Science and Technology Development Foundation, Nanjing Department of Health (grant QYK10135).
We declare that no conflict of interest is present.
Footnotes
Published ahead of print 4 September 2013
REFERENCES
- 1.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368:1888–1897 [DOI] [PubMed] [Google Scholar]
- 2.Ke Y, Wang Y, Liu S, Guo J, Zhang W, Yuan X, Zhang N, Wang Z, Song H, Huang L, Chen Z. 4 July 2013. High severity and fatality of human infections with avian influenza A (H7N9) infection in China. Clinical. Infect. Dis. 10.1093/cid/cit371 [DOI] [PubMed] [Google Scholar]
- 3.Horby P. 2013. H7N9 is a virus worth worrying about. Nature 496:399. [DOI] [PubMed] [Google Scholar]
- 4.Van Ranst M, Lemey P. 2013. Genesis of avian-origin H7N9 influenza A viruses. Lancet 381:1883–1885 [DOI] [PubMed] [Google Scholar]
- 5.Morens DM, Taubenberger JK, Fauci AS. 2013. H7N9 avian influenza A virus and the perpetual challenge of potential human pandemicity. mBio 4(4):e00445-13. 10.1128/mBio.00445-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavia AT. 2013. Influenza A(H7N9): from anxiety to preparedness. Ann. Intern. Med. 159:219–220 [DOI] [PubMed] [Google Scholar]
- 7.Baas C, Barr I, Fouchier R, Kelso A, Hurt A. 2013. A comparison of rapid point-of-care tests for the detection of avian influenza A(H7N9) virus, 2013. Euro Surveill. 18(21):20487 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20487 [PubMed] [Google Scholar]
- 8.Corman VM, Eickmann M, Landt O, Bleicker T, Brunink S, Eschbach-Bludau M, Matrosovich M, Becker S, Drosten C. 2013. Specific detection by real-time reverse-transcription PCR assays of a novel avian influenza A(H7N9) strain associated with human spillover infections in China. Euro Surveill. 18(16):20461 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20461 [PubMed] [Google Scholar]
- 9.Wong CK, Zhu H, Li OT, Leung YH, Chan MC, Guan Y, Peiris JS, Poon LL. 2013. Molecular detection of human H7N9 influenza A virus causing outbreaks in China. Clin. Chem. 59:1062–1067 [DOI] [PubMed] [Google Scholar]
- 10.Chan KH, To KK, Chan JF, Li CP, Chen H, Yuen KY. 2013. Evaluation of analytical sensitivity of seven point-of-care influenza detection kits and two molecular tests for detection of avian-origin H7N9 and swine-origin H3N2 variant influenza A viruses. J. Clin. Microbiol. 51:3160–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63. 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayawardena S, Cheung CY, Barr I, Chan KH, Chen HL, Guan Y, Peiris JSM, Poon LLM. 2007. Loop-mediated isothermal amplification for influenza A (H5N1) virus. Emerg. Infect. Dis. 13:899–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angamuthu R, Baskaran S, Gopal DR, Devarajan J, Kathaperumal K. 2012. Rapid detection of the Marek's disease viral genome in chicken feathers by loop-mediated isothermal amplification. J. Clin. Microbiol. 50:961–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomita N, Mori Y, Kanda H, Notomi T. 2008. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3:877–882 [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI. 2009. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 46:167–172 [DOI] [PubMed] [Google Scholar]
- 16.Njiru ZK. 2012. Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl. Trop. Dis. 6(6):e1572. 10.1371/journal.pntd.0001572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Babiuk S, McIntosh MT. 2012. Development of a loop-mediated isothermal amplification assay for rapid detection of capripoxviruses. J. Clin. Microbiol. 50:1613–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge Y, Wu B, Qi X, Zhao K, Guo X, Zhu Y, Qi Y, Shi Z, Zhou M, Wang H, Cui L. 2013. Rapid and sensitive detection of novel avian-origin influenza A (H7N9) virus by reverse transcription loop-mediated isothermal amplification combined with a lateral-flow device. PLoS One 8:e69941. 10.1371/journal.pone.0069941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui L, Ge Y, Qi X, Xu G, Li H, Zhao K, Wu B, Shi Z, Guo X, Hu L, You Q, Zhang LH, Freiberg AN, Yu X, Wang H, Zhou M, Tang YW. 2012. Detection of severe fever with thrombocytopenia syndrome virus by reverse transcription-cross-priming amplification coupled with vertical flow visualization. J. Clin. Microbiol. 50:3881–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO 8 April 2013. (updated on 15 April 2013) Avian influenza A(H7N9) virus. Real-time RT-PCR protocol for the detection of avian influenza A(H7N9) virus. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/en/
- 21.Nie K, Zhao X, Ding X, Li XD, Zou SM, Guo JF, Wang DY, Gao RB, Li XY, Huang WJ, Shu YL, Ma XJ. 2013. Visual detection of human infection with influenza A (H7N9) virus by subtype-specific reverse transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. Clin. Microbiol. Infect. 19:E372–E375 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, Li Y, Yao X, Zhang Y, Wu H, Zheng S, Diao H, Xia S, Zhang Y, Chan KH, Tsoi HW, Teng JL, Song W, Wang P, Lau SY, Zheng M, Chan JF, To KK, Chen H, Li L, Yuen KY. 2013. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381:1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy A, Cernikova L, Krivda V, Hornickova J. 2012. Digital genotyping of avian influenza viruses of H7 subtype detected in central Europe in 2007-2011. Virus Res. 165:126–133 [DOI] [PubMed] [Google Scholar]
- 24.Kuriakose T, Hilt DA, Jackwood MW. 2012. Detection of avian influenza viruses and differentiation of H5, H7, N1, and N2 subtypes using a multiplex microsphere assay. Avian Dis. 56:90–96 [DOI] [PubMed] [Google Scholar]
- 25.Chan JM, Rabadan R. 2013. Quantifying pathogen surveillance using temporal genomic data. mBio 4(1):e00524-12. 10.1128/mBio.00524-12 [DOI] [PMC free article] [PubMed] [Google Scholar]