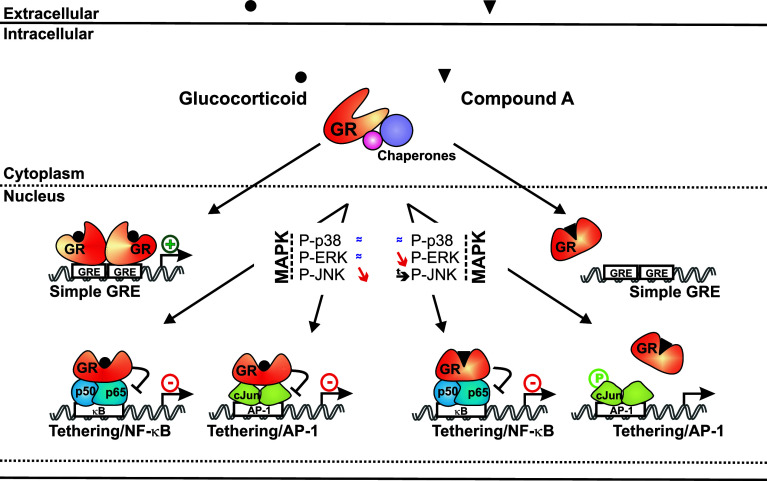
Fig. 11.
Summarizing model for the transcription factor-selective action of CpdA versus DEX. The cytoplasmic GR is kept in a ligand-receptive conformation by binding to chaperone molecules. Upon binding of either GCs or CpdA, the GR changes its specific conformation and translocates into the nucleus. GC-bound GR can form a homodimer and as such binds to a palindromic simple GRE, thus propagating its classic transactivation mechanism. Conversely, CpdA-bound GR cannot form homodimers and is therefore not able to bind a simple GRE or support transactivation. While GC-bound GR leaves p38 and ERK MAPK phosphorylations (almost equal to symbol) unaffected, it diminishes JNK MAPK phosphorylation (slanting down arrow). Stimulation with CpdA affects these MAPK phosphorylations differently, as it actually prolongs JNK MAPK phosphorylation (arrow) and sparks a decline in ERK MAPK phosphorylation (slanting down arrow). Also CpdA does not affect the level of p38 MAPK phosphorylation (almost equal to symbol). As expected from the differential MAPK phosphorylation modulations, CpdA- and GC-bound GRs also show a differentiation in transcription factor targeting. GC-bound GR is recruited onto NF-κB- and AP-1-driven gene promoters and is fully capable of transrepressing both NF-κB- and AP-1-driven gene expression. In contrast, CdpA-bound GR can only bind to NF-κB-driven gene promoters and not to AP-1-driven gene promoters. As such, CpdA-bound GR only supports transrepression of NF-κB-mediated gene transcription, and not AP-1-mediated gene transcription