Abstract
We previously isolated and reported a second species of the Saccharophagus genus, Saccharophagus sp. strain Myt-1. In the present study, a cellulase gene (celMytB) from the genomic DNA of Myt-1 was cloned and characterized. The DNA sequence fragment contained an open reading frame of 1,893 bp that encoded a protein of 631 amino acids with an estimated molecular mass of 66.8 kDa. The deduced protein, CelMytB, had a catalytic domain that contained a conserved signature sequence (VIYEIYNEPL) of glycosyl hydrolase family 5 and a CBM6 cellulose binding module. CelMytB showed optimal activity at 55 °C and pH 6.5, which is similar to the optimal temperature and pH profile of cel5H, an endoglucanase from the closely related S. degradans 2-40. However, the cellulase (degradation of soluble cellulose) and avicelase (degradation of crystalline cellulose) activities of CelMytB were about 3-fold and 100-fold higher, respectively, than the equivalent activities of cel5H. Moreover, CelMytB could degrade xylan. From the zymogram results, we speculated that the catalytic domain of CelMytB had high activity even without the cellulose binding module. The presence of some detergents stimulated the cellulase activity of CelMytB.
Keywords: Cellulase, Saccharophagus sp. Myt-1, Thermophilic, Detergent stable, Xylanase
Introduction
Cellulose is one of the most abundant biomasses and the largest renewable carbon source on Earth [1, 2]. In recent years, cellulases have attracted much attention because of their applications in the food, textile, laundry, pulp and paper industries [3, 4]. In particular, they have been used in degrading agricultural waste and converting it into soluble sugars, alcohol, and other chemical constituents [5, 6]. Because converting cellulosic waste to glucose is globally significant, cellulases are important in biomass use. Thermophilic cellulases specifically are advantageous for many industrial applications because the high processing temperatures they can endure increase the solubility of the reactants and reduce contamination [7]. Therefore, greater understanding of thermophilic cellulases could lead to new and useful industrial applications.
Microbial cellulases from various bacteria have been widely studied [8–10]. One of the most efficient degraders of complex polysaccharides known is Saccharophagus degradans 2-40 [11]. S. degradans 2-40 was first isolated from decaying salt marsh cordgrass collected in the Chesapeake Bay Estuary (USA) [12]. Strain 2-40 is capable of degrading more than 10 complex polysaccharides [13, 14]. To our knowledge, prior to 2012 only one strain of one species had been assigned to the Saccharophagus genus, namely, S. degradans 2-40. Recently, we isolated a novel Saccharophagus species strain Myt-1 from the sediments in Toyama Bay, Japan [15]. Herein, we report the cloning, purification and characterization of the thermophilic cellulase, CelMytB, from strain Myt-1. CelMytB had almost the same thermophilic activity and pH stability as the putative endoglucanase cel5H from S. degradans 2-40. However, the cellulase and avicelase activities of CelMytB were higher than that of cel5H by about 3- and 100-fold, respectively. Moreover, CelMytB could degrade xylan. Further, the presence of some detergents stimulated the cellulase activity of CelMytB.
Materials and Methods
Bacteria and Culture Conditions
Saccharophagus sp. strain Myt-1 was previously isolated from Toyama Bay, Japan [15]. Marine broth medium (Difco marine broth 2216; Becton, Dickinson and Company, MD) was used as the culture medium for the Myt-1 cells.
Cloning and Sequencing the Cellulase Gene
Genomic DNA was isolated from Myt-1 cells using Isoplant II (Nippon Gene, Tokyo, Japan) and partially digested with Sau3AI. The 3–10 kbp DNA fragments were recovered and ligated with the pUC118 vector using a Rapid DNA Dephos and Ligation Kit (Roche, Mannheim, Germany). The ligation products were introduced into E. coli DH5α. To screen for cellulase activity, the transformants were grown on LB agar containing 0.5 % (w/v) carboxymethylcellulose (CMC) and ampicillin (50 μg mL−1) for 1 day at 37 °C, and stained with 0.5 % (w/v) Congo red. We selected the colony with a clear halo and extracted the recombinant plasmid. The inserted DNA fragments were amplified by PCR using the universal M13 primers. The amplified products were purified using a QIAquick PCR kit (Qiagen, Tokyo, Japan) and sequenced directly using the BigDye Terminator v3.1 kit (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 3130xl genetic analyzer (Applied Biosystems). The DNA sequence we obtained was searched against the DNA database using BLAST. The nucleotide and deduced amino acid sequence analyses, open reading frame search, molecular mass and isoelectric point calculations were performed using Genetyx Ver.8 (Genetyx, Tokyo, Japan). The nucleotide sequence of the cellulase gene celMytB determined in this study has been deposited in GenBank/EMBL/DDBJ under accession number AB728496.
Overexpression and Purification of the Recombinant Cellulase CelMytB
The open reading frame of the celMytB was amplified by PCR using the primers 5′-CTCTTTCAGGGACCCGATGTAGCTCCGCTA-3′ (forward) and 5′-CTGCGGCCGCAAGCTTCTACCAGCTACCAAA-3′ (reverse), which were designed based on celMytB. The PCR fragments between the SmaI and HindIII sites of the pET-47b expression vector (Novagen, Madison, WI, USA) were cloned using the In-Fusion Advantage PCR Cloning Kit (Takara Bio, Shiga, Japan). This recombinant plasmid was used for expression of the cellulase gene in E. coli BL21 (DE3) [16]. The purity of the protein was examined by SDS-PAGE [17]. The gels were stained for proteins using Quick Blue Staining Solution (Bio Dynamics Laboratory Inc., Tokyo, Japan) and the molecular mass of the resulting bands was estimated using high-range or broad molecular mass standards (Bio-Rad Laboratories Inc., Tokyo, Japan). To detect cellulase and xylanase activity, we used SDS–polyacrylamide gels containing 0.1 % (w/v) CMC and 0.2 % (w/v) xylan, respectively. Following electrophoresis, active bands were detected with Congo red [18, 19].
Characterization of Cellulase
Cellulase activity was quantified by measuring the quantity of reducing sugars following reaction by the Somogyi–Nelson method [20, 21]. One unit (U) of enzyme activity was defined as the amount of protein required to release an amount of reducing sugars equivalent to 1 μmol of glucose per min. The optimal temperature and pH of the purified cellulase was determined in 50 mM GTA buffer containing 1 % (w/v) CMC for 30 min. The effects of detergents on cellulase activity were analyzed by measuring the activity of the enzyme at 55 °C in 50 mM GTA buffer (pH 6.5) containing 0.001, 0.01 or 0.1 % (w/v) of selected detergents. To determine the substrate specificity of the cellulase, the enzyme assay was performed in a reaction mixture containing 1 % (w/v) each of CMC, xylan and Avicel in GTA buffer (pH 6.5) for 30 min at 55 °C. The reducing sugars were released into the solution and measured using the dinitrosalicylic acid assay [22].
Results and Discussion
Cloning and Sequence Analysis of Cellulase Gene celMytB
The enzymes and gene resources of microorganisms have drawn considerable attention for their potential values in industry and biotechnology. To date, S. degradans 2-40 is the most efficient carbohydrate processing strain known, capable of degrading more than 10 complex polysaccharides [11]. In fact, some of the unique polysaccharide degrading enzymes from strain 2-40 have been cloned and characterized [23, 24]. We previously isolated a novel Saccharophagus species strain Myt-1 from Toyama Bay in Japan [15], and in this study we cloned a cellulase gene celMytB from strain Myt-1 and characterized the cellulase (CelMytB) that it encodes.
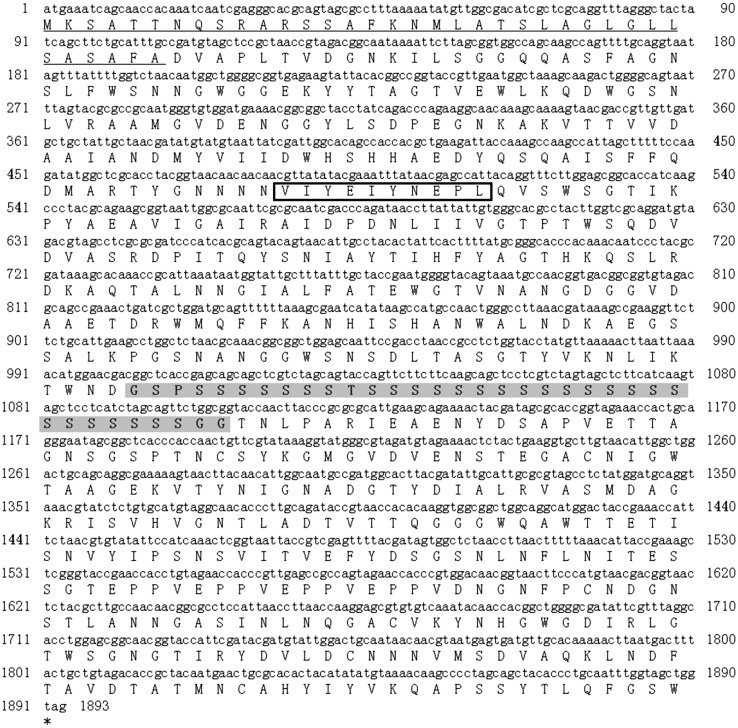
One positive clone for cellulase was isolated from the strain Myt-1 genomic library. Insert DNA fragments of about 2.3 kbp were detected by agarose gel electrophoresis and sequenced. Sequence analysis of the fragments revealed the presence of a single open reading frame of 1,893 bp, which we designated celMytB (Fig. 1). The celMytB gene encoded a protein of 631 amino acids with an estimated molecular mass of 66.8 kDa and a pI of 4.63. Genetyx Ver.8 predicted that this protein possessed a typical signal peptide with a cleavage site between Ala36 and Asp37 at the protein’s N-terminal. In addition, the conserved signature sequence of glycosyl hydrolase family 5 (GH5) (VIYEIYNEPL) [25] was identified in CelMytB, suggesting that CelMytB was a member of the GH5 family. A catalytic domain and carbohydrate binding module (CBM) were predicted from the primary structure of CelMytB on the NCBI website (http://www.ncbi.nlm.nih.gov). The CBM was classified as belonging to the CBM6 family. The molecular mass of the catalytic domain and CBM were about 30 and 25 kDa, respectively. A polyserine linker (PSL) that had glycine residues at its N- and C-terminal ends was identified in the CelMytB sequence (Fig. 1). Howard et al. [26] reported that the PSL acted as a flexible link between catalytic and binding domains. Badieyan et al. [27] found that protein flexibility and optimum activity at high temperatures were appreciably correlated. Thus, a highly flexible CelMytB protein molecule is predicted to be active at high temperatures. A BLAST homology search indicated that CelMytB shared an amino acid sequence identity of 99.4 % with the Saccharophagus sp. JAM-R001 putative endoglucanase (egl-R1; BAI50016), 98.9 % with S. degradans 2-40 putative endoglucanase (cel5H; ABD82494), and 75.7 % with Cellvibrio japonicus Ueda107 endo-1,4-beta glucanase (cel5B; ACE84076). However, although CelMytB and the endoglucanase egl-R1 from Saccharophagus sp. JAM-R001 had the highest similarity, JAM-R001 was identified from a metagenome and was not an isolated strain, and egl-R1 has not been characterized by either biochemical or physiological analysis.
Fig. 1.
Nucleotide and deduced amino acid sequences of celMytB gene from Saccharophagus sp. Myt-1. The predicted signal peptide is underlined. The highly conserved catalytic domain signature of glycosyl hydrolase family 5 (GH5) is boxed. The polyserine linker is highlighted in gray
Expression and Purification of CelMytB
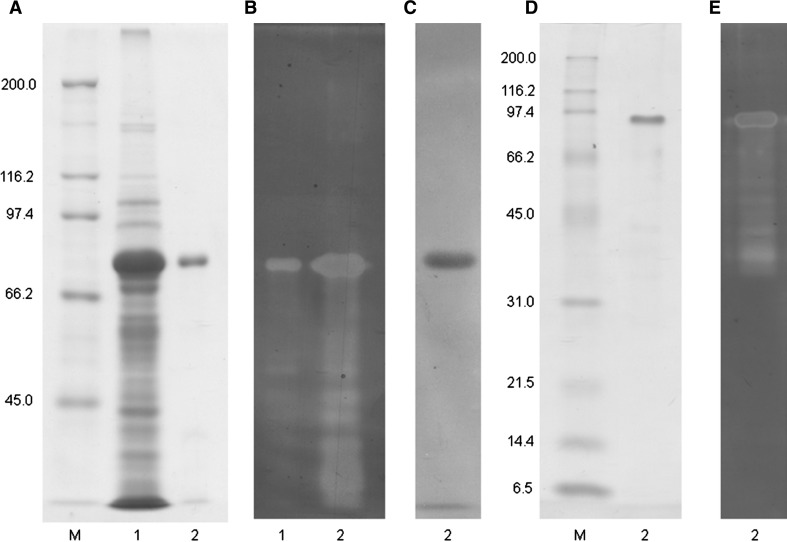
The celMytB gene, excluding the region that codes the putative signal peptide sequence, was cloned into the pET-47b (+) vector, which was then used to transform E. coli BL21 (DE3). The enzyme was overexpressed as an N-terminal His-tagged protein with an estimated molecular mass of 76.8 kDa. After IPTG induction, the enzyme was purified from the cell extract using a His GraviTrap column and then refolded by fractional dialysis. The purified CelMytB gave a single band with an apparent molecular mass of 76.8 kDa on SDS-PAGE (Fig. 2a, d). However, a zymogram analysis of 15 % acrylamide gels detected many lower bands between about 30 and 76.8 kDa (Fig. 2b, e), while no bands lower than about 30 kDa were found (Fig. 2e). This made us speculate that if the CelMytB protein had denatured or cleaved during SDS-PAGE, the activity of CelMytB would remain until the catalytic domain was denatured, suggesting that the catalytic domain of CelMytB has strong activity even without a functional cellulose binding module.
Fig. 2.
SDS-PAGE and zymogram analysis of purified recombinant cellulase CelMytB from Saccharophagus sp. Myt-1. a 7.5 % gel with Coomassie blue staining. b 7.5 % gel with active staining for cellulose. c 7.5 % gel with active staining for xylan. d 15 % gel with Coomassie blue staining. e 15 % gel with active staining for cellulose. M protein markers, 1 crude protein extract, 2 purified cellulase
Effects of Temperature, pH and Various Detergents on Activity and Stability of CelMytB
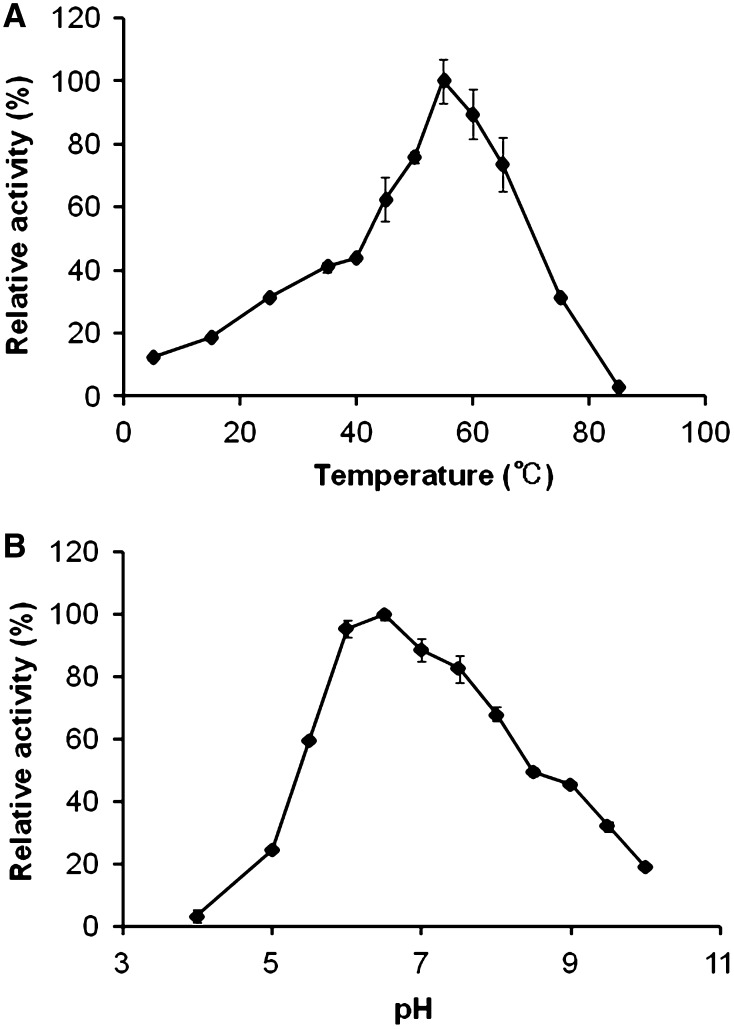
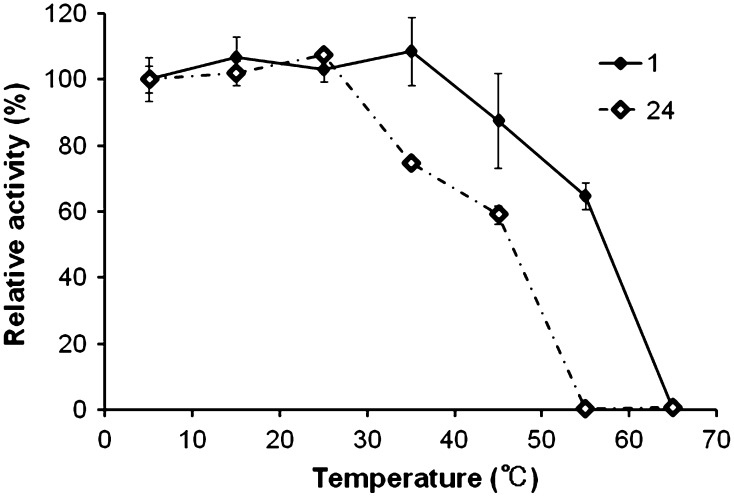
The effect of temperature on CelMytB activity was determined from 5 to 85 °C, with 55 °C being the optimum temperature for maximum enzyme activity (1,800 ± 67 U/μmol enzyme) while about 30 % of maximal activity was retained at 25 and 75 °C (Fig. 3a). CelMytB was most active at pH 6.5 (2,000 ± 38 U/μmol enzyme) and retained more than 90 % of its activity between pH 6.0 and 7.0 (Fig. 3b), and more than 20 % of its activity still remained at pH 5.0 and 10.0. The optimal temperature and pH of CelMytB were approximately the same as those of cel5H from S. degradans 2-40 [24]. We found that CelMytB retained 100 % of its activity when incubated at 45 °C for 1 h (Fig. 4). Moreover, CelMytB retained 100 and 60 % of its activity when incubated at 25 and 45 °C for 24 h, respectively. The thermal stabilities of egl-R1 and cel5H were not reported. These results suggest that CelMytB is a thermophilic, thermostable and pH stable cellulase, and hence may be useful in industries that use extreme thermal conditions. CelMytB activity was very stable and was stimulated in the presence of 0.001 or 0.01 % Tween40 and 0.001 % TritonX-100 (Table 1). Also, the effects of these detergents on egl-R1 and cel5H activities have not been reported until now (Table 2). These results suggest that CelMytB may be useful in industries that employ detergents.
Fig. 3.
Effects of temperature and pH on recombinant CelMytB activity. a CelMytB mixtures were incubated at temperatures from 5 to 85 °C for 30 min in GTA buffer (pH 6.5). The activity of the purified CelMytB at the optimum temperature of 55 °C was taken as 100 %. b CelMytB mixtures were incubated at 55 °C for 30 min in GTA buffer at pHs from 4.0 to 10.0. The activity of the purified CelMytB at the optimum pH of 6.5 was taken as 100 %
Fig. 4.
Thermostability of CelMytB at different temperatures. CelMytB was preincubated at different temperatures (5–65 °C) for 1 or 24 h, and then the residual activity was measured. The activity of the purified cellulase at 5 °C (1,900 ± 130 U/μmol enzyme) was taken as 100 %
Table 1.
Effect of various detergents on CelMytB activity
| Detergents | Concentration (%) | Relative activitya (%) |
|---|---|---|
| No addition | 0 | 100.0 ± 3.6 |
| SDS | 0.001 | 107.8 ± 8.0 |
| 0.01 | 94.4 ± 3.7 | |
| 0.1 | 92.8 ± 4.1 | |
| Tween20 | 0.001 | 109.5 ± 1.7 |
| 0.01 | 98.0 ± 1.3 | |
| 0.1 | 104.0 ± 6.7 | |
| Tween40 | 0.001 | 124.3 ± 15.9 |
| 0.01 | 116.2 ± 2.8 | |
| 0.1 | 110.5 ± 1.2 | |
| Tween80 | 0.001 | 111.3 ± 9.8 |
| 0.01 | 107.6 ± 6.7 | |
| 0.1 | 89.0 ± 8.0 | |
| TritonX-100 | 0.001 | 122.2 ± 0.1 |
| 0.01 | 105.7 ± 5.7 | |
| 0.1 | 90.1 ± 6.4 |
aThe activity was determined by the Somogyi–Nelson method
Table 2.
Comparison of the properties of CelMytB with cellulases from other closely related Saccharophagus species
| Characteristics | CelMytB | egl-R1 | cel5H |
|---|---|---|---|
| Similarity (amino acid) (%) | – | 99.4 | 98.9 |
| Molecular mass (kDa) | 66.8 | 66.9 | 66.9 |
| Optimum temperature (°C) | 55 | ND | 50 |
| Optimum pH | 6.5 | ND | 6.5 |
| Thermal stability | Retained 100 and 60 % of its activity when incubated for 24 h at 25 and 45 °C, respectively | ND | ND |
| Effects of detergents | |||
| Stimulated | Tween40, TritonX-100 | ND | ND |
| Not affected | SDS, Tween20, Tween80 | ND | ND |
| Specific activity of enzymes on various substrates (U/μmol enzyme) | |||
| CMC | 1,800 ± 67 | ND | 643 ± 148 |
| Avicel | 130 ± 10 | ND | 1.45 ± 0.1 |
| Xylan | 25 ± 5 | ND | ND |
ND not determined
Substrate Specificity of CelMytB
Asha et al. [28] reported that cellulase from Paenibacillus barcinonensis could degrade various cellulosic substrates such as CMC, Avicel and xylan. For comparison, the specificity of CelMytB for these substrates was examined and activities of 1,800 ± 67, 130 ± 10 and 25 ± 5 U/μmol enzyme for CMC, Avicel and xylan, respectively, were obtained. The cellulase and avicelase activities of CelMytB were about 3- and 100-fold higher, respectively, than those of cel5H [24]. In addition, xylanase activity was detected by the dinitrosalicylic acid assay and confirmed by the zymogram (Fig. 2c). This study is the first to find xylanase activities of cellulase from Saccharophagus sp. Xylanases have also attracted considerable attention because of their application in various industrial processes such as pulp, brewing, feed and food [29]. CelMytB, therefore, has potential industrial applications because it can degrade not only soluble cellulose (CMC), but also crystalline cellulose (Avicel) and xylan.
Conclusion
We characterized a recombinant cellulase CelMytB from Saccharophagus sp. strain Myt-1 and found its unique characteristics. The catalytic domain of CelMytB may have significant activity even without the cellulose binding module. CelMytB exhibited high activity at high temperature over a wide pH range. At high temperatures CelMytB exhibited good stability and high cellulase activity. Moreover, we found that CelMytB can degrade soluble cellulose, crystalline cellulose and xylan. These properties and the stability of CelMytB in the presence of several detergents indicate that this enzyme has potential applications in the food, textile, laundry, pulp and paper industries [30].
Acknowledgments
This study was partly supported by grants from the Japan Society for the Promotion of Science (Grant-in Aid for JSPS Research Fellows No. 10085 (to AS).
References
- 1.Jarvis M. Chemistry: cellulose stacks up. Nature. 2003;426:611–612. doi: 10.1038/426611a. [DOI] [PubMed] [Google Scholar]
- 2.Jung YJ, Lee YS, Park IH, Chandra MS, Kim KK, Choi YL. Molecular cloning, purification and characterization of thermostable β-1,3-1,4 glucanase from Bacillus subtilis A8-8. Indian J Biochem Biophys. 2010;47:203–210. [PubMed] [Google Scholar]
- 3.Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000;18:355–383. doi: 10.1016/S0734-9750(00)00041-0. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Kim BK, Lee BH, Jo KI, Lee NK, Chung CH, Lee YC, Lee JW. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour Technol. 2008;99:378–386. doi: 10.1016/j.biortech.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng IS, Li CW, Yeh YF, Chen PT, Chir JL, Ma CH, Yu SM, Ho TH, Tong CG. A novel endo-glucanase from the thermophilic bacterium Geobacillus sp. 70PC53 with high activity and stability over a broad range of temperatures. Extremophiles. 2009;13:425–435. doi: 10.1007/s00792-009-0228-4. [DOI] [PubMed] [Google Scholar]
- 8.Garsoux G, Lamotte J, Gerday C, Feller G. Kinetic and structural optimization to catalysis at low temperatures in a psychrophilic cellulase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Biochem J. 2004;384:247–253. doi: 10.1042/BJ20040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Wang HL, Li T, Yu HY. Purification and characterization of an organic solvent-tolerant alkaline cellulase from a halophilic isolate of Thalassobacillus. Biotechnol Lett. 2012;34:1531–1536. doi: 10.1007/s10529-012-0938-z. [DOI] [PubMed] [Google Scholar]
- 10.Vijayaraghavan P, Vincent SG. Purification and characterization of carboxymethyl cellulase from Bacillus sp. isolated from a paddy field. Pol J Microbiol. 2012;61:51–55. [PubMed] [Google Scholar]
- 11.Ekborg NA, Gonzalez JM, Howard MB, Taylor LE, Hutcheson SW, Weiner RM. Saccharophagus degradans gen. nov., sp. nov., a versatile marine decomposer of complex polysaccharides. Int J Syst Evol Microbiol. 2005;55:1545–1549. doi: 10.1099/ijs.0.63627-0. [DOI] [PubMed] [Google Scholar]
- 12.Andrykovitch G, Marx I. Isolation of a new polysaccharide-digesting bacterium from a salt marsh. Appl Environ Microbiol. 1988;54:1061–1062. doi: 10.1128/aem.54.4.1061-1062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González JM, Weiner RM. Phylogenetic characterization of marine bacterium strain 2-40, a decomposer of complex polysaccharides. Int J Syst Evol Microbiol. 2000;50:831–834. doi: 10.1099/00207713-50-2-831. [DOI] [PubMed] [Google Scholar]
- 14.Weiner RM, Taylor LE, II, Henrissat B, et al. Complete genome sequence of the complex carbohydrate-degrading marine bacterium, Saccharophagus degradans strain 2-40 T. PLoS Genet. 2008;4:e1000087. doi: 10.1371/journal.pgen.1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakatoku A, Wakabayashi M, Tanaka Y, Tanaka D, Nakamura S. Isolation of a novel Saccharophagus species (Myt-1) capable of degrading a variety of seaweeds and polysaccharides. MicrobiologyOpen. 2012;1:2–12. doi: 10.1002/mbo3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka D, Yoneda S, Yamashiro Y, Sakatoku A, Kayashima T, Yamakawa K, Nakamura S. Characterization of a new cold-adapted lipase from Pseudomonas sp. TK-3. Appl Biochem Biotechnol. 2012;168:327–338. doi: 10.1007/s12010-012-9776-7. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz WH, Bronnenmeier K, Gräbnitz F, Staudenbauer WL. Activity staining of cellulases in polyacrylamide gels containing mixed linkage β-glucans. Anal Biochem. 1987;164:72–77. doi: 10.1016/0003-2697(87)90369-1. [DOI] [PubMed] [Google Scholar]
- 19.Ratanakhanokchai K, Kyu KL, Tanticharoen M. Purification and properties of a xylan-binding endoxylanase from Alkaliphilic Bacillus sp. strain K-1. Appl Environ Microbiol. 1999;65:694–697. doi: 10.1128/aem.65.2.694-697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1994;153:375–380. [Google Scholar]
- 21.Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- 22.Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- 23.Ko JK, Jung MW, Kim KH, Choi IG. Optimal production of a novel endo-acting beta-1,4-xylanase cloned from Saccharophagus degradans 2-40 into Escherichia coli BL21 (DE3) N Biotechnol. 2009;26:157–164. doi: 10.1016/j.nbt.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Watson BJ, Zhang H, Longmire AG, Moon YH, Hutcheson SW. Processive endoglucanases mediate degradation of cellulose by Saccharophagus degradans. J Bacteriol. 2009;191:5697–5705. doi: 10.1128/JB.00481-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z, Ruan L, Chen X, Zhang Y, Xu X. A novel salt-tolerant endo-beta-1,4-glucanase Cel5A in Vibrio sp. G21 isolated from mangrove soil. Appl Microbiol Biotechnol. 2010;87:1373–1382. doi: 10.1007/s00253-010-2554-y. [DOI] [PubMed] [Google Scholar]
- 26.Howard MB, Ekborg NA, Taylor LE, Hutcheson SW, Weiner RM. Identification and analysis of polyserine linker domains in prokaryotic proteins with emphasis on the marine bacterium Microbulbifer degradans. Protein Sci. 2004;13:1422–1425. doi: 10.1110/ps.03511604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badieyan S, Bevan DR, Zhang C. Study and design of stability in GH5 cellulases. Biotechnol Bioeng. 2012;109:31–44. doi: 10.1002/bit.23280. [DOI] [PubMed] [Google Scholar]
- 28.Asha BM, Revathi M, Yadav A, Sakthivel N. Purification and characterization of a thermophilic cellulase from a novel cellulolytic strain, Paenibacillus barcinonensis. J Microbiol Biotechnol. 2012;22:1501–1509. doi: 10.4014/jmb.1202.02013. [DOI] [PubMed] [Google Scholar]
- 29.Beg QK, Kapoor M, Mahajan L, Hoondal GS. Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- 30.Kuhad RC, Gupta R, Singh A. Microbial cellulases and their industrial applications. Enzym Res. 2011 doi: 10.4061/2011/280696. [DOI] [PMC free article] [PubMed] [Google Scholar]