Fig. 3.
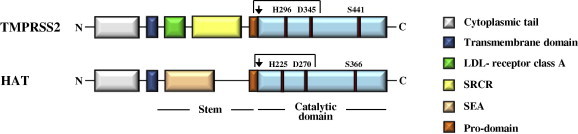
Domain organization of TMPRSS2 and HAT. TMPRSS2 and HAT, both members of the type II transmembrane serine protease family, contain an N-terminal cytoplasmic domain, a transmembrane domain, a stem region and a catalytic domain. The stem region of TMPRSS2 harbors a LDL-receptor class A domain and a scavenger receptor cysteine-rich domain (SRCR), while sperm protein, enterokinase and agrin (SEA) domain is present in the stem region of HAT. The catalytic domains of both proteases contain a catalytic triad, consisting of a serine (S), histidine (H) and aspartate (D) residue, which is essential for enzymatic activity. Both enzymes are synthesized as inactive precursors, zymogens, and transit into their active form upon cleavage between the pro- and catalytic domain (indicated by an arrow). In the mature enzyme, the catalytic domain and the remainder of the protein covalently associated via a disulfide bond.
