Abstract
The purpose of this study was to compare the prevalence of severe sarcopenia detected by total skeletal muscle mass (SM) index and of site-specific thigh sarcopenia for differing age groups in men and women. Japanese nonobese men and women aged 20 to 85 (n = 1,994, 55 % women) had muscle thickness (MTH) measured by ultrasound at six sites on the anterior and posterior aspects of the body. SM was estimated from ultrasound-derived prediction equations. Site-specific thigh sarcopenia was calculated using ultrasound-measured MTH at the anterior and posterior aspects of the thigh (MTH ratio, anterior 50 %/posterior 50 % thigh MTH (A50/P50 MTH)). Sarcopenia was defined as a SM index (SM divided by height2) of >2 standard deviations (SD) below the mean for young adults. Site-specific thigh sarcopenia was defined as a ratio of A50/P50 MTH of >2 SD below the mean for young adults. Age was inversely correlated to SM index and A50/P50 MTH in men (r = −0.480 and r = −0.522) and women (r = −0.243 and r = −0.516). The prevalence rate of sarcopenia was less than 3 % for women under the age of 60, 7 % for ages 60–69, and 24 % for ages 70–80. In men, the prevalence rate of sarcopenia was less than 7 % under the age of 50, 18 % for ages 50–59, 33 % for ages 60–69, and 47 % for ages 70–85. Compared to the sarcopenia estimated by SM index, there was a higher prevalence of site-specific thigh sarcopenia observed in both sexes. These results suggest that site-specific thigh sarcopenia appears before it is able to be detected at the whole body level.
Keywords: Aging, Skeletal muscle mass, Ultrasound, Thigh muscle thickness
Introduction
The proportion of time spent performing a particular mode of physical activity varies according to age (Belanger et al. 2011). Exercise and fitness activities including team sports make up a greater proportion of total moderate or vigorous physical activity time in young adults, whereas domestic activities make up a greater proportion of total moderate/vigorous physical activity time in older adults (Belanger et al. 2011). These daily physical activities include sports that are highly dependent upon age-related changes in skeletal muscle mass (Park et al. 2010; Abe et al. 2012b). A limited number of studies have reported that the electromyogram activity patterns during daily activities differed between muscle groups located in the anterior and posterior aspects of the limbs (Sawai et al. 2006; Shiraiwa et al. 2009). Recent studies have demonstrated that an age-related site-specific loss of skeletal muscle mass is observed in Japanese (Abe et al. 2011b) and German (Abe et al. 2011a) populations, especially for the anterior thigh muscles (Ogawa et al. 2012). Similarly, one longitudinal study found a significant reduction in mid-thigh muscle cross-sectional area (CSA), while the posterior muscle CSA did not change (Frontera et al. 2008). In addition, a recent study reported that the age-related loss of the anterior thigh muscle is inversely associated with zigzag walking performance, but not with normal and maximum walking speed in middle-aged and older women (Abe et al. 2012c). These results suggest that site-specific muscular changes begin appearing before they are able to be detected at the whole body level.
Based on the definition of sarcopenia by Baumgartner et al. (appendicular lean mass index (aLM index), <7.26 kg/m2 for men and <5.45 kg/m2 for women), approximately 14 % of non-Hispanic men under 70 years of age, 20 % for those between 70 and 74 years, 27 % for those between 75 and 79 years, and 53 % for those aged over 80 years were classified as being sarcopenic (Baumgartner et al. 1998). Using the same diagnostic criteria, the prevalence of sarcopenia was approximately 4 % for European men under the age of 59 years, 4 % for those between 60 and 69 years, and 7 % for those over 70 years of age (Bijlsma et al. 2013). On the other hand, sarcopenic obesity is the combination of reduced skeletal muscle mass and increased fat mass with advancing age. A study reported that the prevalence of sarcopenic obesity ranged from 4 to 12 % in older men and women (Stenholm et al. 2008). The prevalence of sarcopenia as well as sarcopenic obesity indicates a pattern that increases with age, although the rate varies widely in the literature. Skeletal muscle mass in later life is determined by both peak muscle mass attained in early life and the rate of muscle loss. Thus, higher muscle mass may present an advantage for delaying or preventing sarcopenia; however, little is known of the prevalence rates for low levels (>2 standard deviations (SD) below of reference value) of muscle mass in young and middle-aged men and women. Newman and Kupelian (2003) demonstrated relationships between aLM index and anthropometric variables and found that the aLM is strongly correlated (men, r = 0.76 [n = 1,435]; women, r = 0.85 [n = 1,549]) with body mass index (BMI). In addition, the prevalence rate of sarcopenia in men and women was higher in those with low BMI than those with high BMI values. Mean values for BMI are generally increased with age in both sexes (Guo et al. 1999; Livshits et al. 2012); therefore, when evaluating for prevalence of sarcopenia, similar BMI ranges should be considered to compare among different age groups. To the best of our knowledge, the prevalence of site-specific sarcopenia in young, middle-aged, and older people has not been reported. In the present study, we compare the prevalence of severe sarcopenia detected by total skeletal muscle mass index and of site-specific thigh sarcopenia for different age groups in men and women.
Methods
Subjects
Subjects were recruited from participants who had received a community-based general health examination (age ≥35 years). Younger adults (age 20–34 years) were recruited through printed advertisements and by word of mouth from the university campus and surrounding area. Prior to obtaining informed consent, a written description of the purpose of the study and its safety was distributed to potential subjects. All subjects were free of overt chronic disease (e.g., diabetes, angina, myocardial infarction, cancer, stroke, etc.) as assessed by self-report. In addition, according to the World Health Organization, obesity is defined as body mass index ≥30 kg/m2 (WHO 2000), subjects with a higher body mass index (≥30 kg/m2) were also excluded in order to increase the similarity of BMIs between groups and limit the influence of adiposity on muscle mass. As a result, 896 men and 1,098 women aged 20 to 85 years were used for data analyses (Table 1). This study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee for Human Experiments of the academic institute.
Table 1.
Body composition of men and women in each age group
| Age group (years) | |||||||
|---|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–85 | p value | |
| Men (n = 896) | |||||||
| N | 64 | 119 | 206 | 176 | 252 | 79 | |
| Age, years | 25 (3)abcde | 36 (3)fbcde | 44 (3)facde | 55 (3)fabde | 64 (3)fabce | 74 (4)fabcd | <0.001 |
| Height, cm | 171 (6)bcde | 169 (5)bcde | 166 (6)facde | 160 (6)fabde | 158 (6)fabc | 157 (5)fabc | <0.001 |
| Body mass, kg | 67.7 (9.8)bcde | 65.8 (8.1)cde | 64.4 (8.0)fcde | 59.1 (8.1)fabde | 55.1 (8.2)fabc | 53.8 (7.7)fabc | <0.001 |
| BMI, kg/m2 | 23.2 (2.7)de | 23.1 (2.6)de | 23.3 (2.4)de | 23.0 (2.6)de | 22.1 (2.6)fabc | 21.9 (2.7)fabc | <0.001 |
| Body fat, % | 18.2 (5.8) | 18.7 (4.7)cde | 18.1 (3.5)cde | 17.0 (3.2)ab | 16.4 (3.2)ab | 16.7 (3.4)ab | <0.001 |
| FFM, kg | 55.0 (6.1)bcde | 53.2 (4.9)cde | 52.5 (5.4)fcde | 48.9 (5.8)fabde | 45.9 (5.7)fabc | 44.7 (5.2)fabc | <0.001 |
| Women (n = 1,098) | |||||||
| N | 86 | 88 | 179 | 316 | 342 | 87 | |
| Age, years | 23 (3)abcde | 36 (3)fbcde | 45 (3)facde | 55 (3)fabde | 64 (3)fabce | 73 (3)fabcd | <0.001 |
| Height, cm | 159 (5)abcde | 154 (6)fcde | 153 (5)fcde | 149 (5)fabde | 147 (5)fabce | 144 (5)fabcd | <0.001 |
| Body mass, kg | 54.0 (6.5)de | 52.5 (7.0)e | 54.8 (7.9)de | 53.0 (7.7)e | 51.3 (7.5)fbe | 47.1 (7.8)fabcd | <0.001 |
| BMI, kg/m2 | 21.3 (2.2)bcde | 22.1 (2.7)bcd | 23.4 (3.2)fa | 23.8 (3.0)fa | 23.8 (3.2)fa | 22.7 (3.2)f | <0.001 |
| Body fat, % | 24.1 (4.8)e | 23.4 (3.9) | 24.8 (4.3)e | 24.7 (4.1)e | 24.1 (4.2)e | 21.7 (4.6)fbcd | <0.001 |
| FFM, kg | 40.8 (3.7)de | 40.0 (4.2)e | 41.0 (4.3)cde | 39.7 (4.5)bde | 38.7 (4.4)fbce | 36.6 (4.4)fabcd | <0.001 |
Significance was set at p < 0.05
BMI body mass index, FFM fat-free mass
aSignificant group difference for ages 30–39
bSignificant group difference for ages 40–49
cSignificant group difference for ages 50–59
dSignificant group difference for ages 60–69
eSignificant group difference for ages 70–85
fSignificant group difference for ages 20–29
Skeletal muscle mass and thigh muscle thickness ratio
Total skeletal muscle mass (SM) was estimated from ultrasound-derived prediction equations that converted muscle thickness (MTH) to SM (Sanada et al. 2006). A strong correlation (R2 = 0.94) has previously been observed between magnetic resonance imaging-measured total SM and ultrasound-predicted total SM. Recently, we examined the relationship between dual-energy X-ray absorptiometry (DXA)-estimated aLM and total SM predicted by ultrasound and found that there is a strong correlation (R2 = 0.95) between the two methods (unpublished observation). MTH was measured using B-mode ultrasound (Aloka SSD-500, Tokyo, Japan) at six sites on the anterior and posterior aspects of the body (upper arm, trunk, and thigh) as previously described (Abe et al. 1994). The measurements were taken while the subjects stood with their elbows and knees extended and relaxed because MTH for the prediction equation of SM was measured in a standing position. Muscle tone changes in the supine position, and there are significant differences in MTH between standing and the supine position (Abe et al. 1997). A 5-MHz scanning head was placed on the measurement site without depressing the dermal surface. The subcutaneous adipose tissue–muscle interface and the muscle–bone interface were identified from the ultrasonic image, and the distance between two interfaces was recorded as MTH. To evaluate site-specific sarcopenia of the thigh, the ratio of anterior and posterior thigh MTH (anterior 50 %/posterior 50 % thigh MTH (A50/P50 MTH)) was calculated.
Body composition and anthropometry
Subcutaneous fat thickness was measured using ultrasound at six sites, as described previously (Abe et al. 1994). Body density was estimated from subcutaneous fat thickness using an ultrasound-derived prediction equation (Abe et al. 1994). Percent body fat (%fat) was calculated from body density using Brozek’s equation (Brozek et al. 1963). We have reported previously that the standard error of the estimate of body density calculated using the ultrasound equations is approximately 0.006 g/mL (or an error of about 2.5 %fat) in a normal-weight Japanese population (Abe et al. 1994). Fat-free mass (FFM) was estimated as total body mass minus fat mass. Body mass and standing height were measured to the nearest 0.1 kg and 0.1 cm, respectively, by using an electronic weight scale and a stadiometer. BMI was defined as body mass/height2 (in kilograms per square meter).
Definition of sarcopenia detected by whole body muscle mass
Severe sarcopenia was defined as a skeletal muscle mass index (SM/height2, SM index) value of 2 SD below the mean for young adults. In a previous study, we reported a SM index of Japanese men aged 20 to 30 years (8.6 [SD 0.9] kg/m2) as the reference value for men (Abe et al. 2012d). For women, we recalculated a mean and SD of young women aged 20–30 years (6.0 [SD 0.9] kg/m2) as the reference value using a previously reported data set (Abe et al. 2012a). Therefore, the reference values for severe sarcopenia (2 SD below the sex-specific means) in men and women were 6.8 and 4.2 kg/m2, respectively.
Definition of site-specific thigh sarcopenia
Site-specific sarcopenia was defined as a thigh muscle thickness ratio (A50/P50 MTH) value of 2 SD below the mean for young adults. Because there are no published site-specific reference values for young adults, we used a mean and SD from the current study (aged 20–29 years) for diagnostic criteria of site-specific thigh sarcopenia (0.91 [0.12] for men and 0.91 [0.11] for women). Therefore, the reference values for site-specific thigh sarcopenia (2 SD below the sex-specific means) in men and women were 0.67 and 0.69, respectively.
Statistical analysis
Results are expressed as means and SD. The differences between age groups for age, height, body mass, BMI, percent body fat, fat-free mass, SM, SM index, A50 MTH, P50 MTH, and A50/P50 MTH were tested for significance by one-way analysis of variance, followed by pairwise comparisons using Tukey’s multiple comparison procedure if a significant F test was obtained. If variances were unequal, Dunnett’s C procedure was performed. Pearson product correlations were performed to determine the relationships between age and total SM and SM index and between age and thigh muscle thickness (A50/P50 MTH) ratio. Subjects were classified as having severe sarcopenia based on SM index as well as site-specific thigh sarcopenia based on A50/P50 MTH ratio. p values <0.05 were considered statistically significant.
Results
Age-related change in body composition and muscle mass
There were an unequal number of subjects in each age group for men and women, and the number of subjects in ages 20–29 as well as ages 70–85 was relatively smaller than that of the other age groups in both sexes. BMI was similar among younger (ages 20–29 and 30–39) and middle-aged (ages 40–49 and 50–59) men and was lower in older men (ages 60–69 and 70–85) compared with the younger and middle-aged men. For women, BMI was similar between ages 20–29 and 30–39 and was higher in middle-aged and older groups than in the younger groups. In men, %fat was lower in ages 50–59 and older groups (ages 60–69 and 70–85) compared with ages 30–39 and 40–49. On the other hand, %fat in women was similar among age groups, except ages 70–85 where it was lower. FFM gradually decreased with age for men and was lower in older men (ages 60–69 and 70–85) compared with the younger and middle-aged men. Similarly, FFM was lower in older women than in younger and middle-aged women (Table 1).
Anterior thigh (A50) MTH as well as SM gradually decreased with age in men and women, except between ages 30–39 and 40–49, which was similar in women. On the other hand, posterior thigh (P50) MTH was similar among younger (ages 20–29 and 30–39) and middle-aged (ages 40–49 and 50–59) men. In women, P50 MTH was similar among ages 30–39, 40–49, and 50–59 and was higher in these age groups than that of ages 20–29 and 70–85.
In men, SM index and A50/P50 MTH ratio gradually decreased with age. Compared to the youngest men (ages 20–29), SM index was 11 and 22 % lower in the age groups 50–59 and 70–85, respectively. Similarly, A50/P50 MTH ratio was 25 and 31 % lower in the age groups 50–59 and 70–85, respectively, compared to the youngest men. On the other hand, SM index was similar among younger (ages 20–29 and 30–39) and ages 40–49 women and was lower in older women than in the young and middle-aged women. A50/P50 MTH ratio gradually decreased with age in women (Table 2).
Table 2.
Muscle mass and thigh muscle thickness ratio of men and women in each age group
| Age group (years) | |||||||
|---|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–85 | p value | |
| Men (n = 896) | |||||||
| N | 64 | 119 | 206 | 176 | 252 | 79 | |
| SM, kg | 25.0 (3.4)abcde | 23.5 (2.9)fbcde | 22.4 (2.8)facde | 19.7 (2.9)fabde | 18.0 (2.8)fabce | 16.5 (3.0)fabcd | <0.001 |
| SM index, kg/m2 | 8.57 (0.98)bcde | 8.26 (0.89)cde | 8.10 (0.86)fcde | 7.66 (1.03)fabde | 7.24 (0.98)fabce | 6.68 (1.08)fabcd | <0.001 |
| A50 MTH, cm | 5.42 (0.62)abcde | 4.95 (0.72)fbcde | 4.61 (0.65)facde | 3.96 (0.64)fabde | 3.77 (0.58)fabce | 3.37 (0.59)fabcd | <0.001 |
| P50 MTH, cm | 5.99 (0.65)de | 5.94 (0.61)de | 5.91 (0.63)de | 5.78 (0.74)de | 5.54 (0.75)fabc | 5.44 (0.85)fabc | <0.001 |
| A50/P50 MTH ratio | 0.91 (0.12)abcde | 0.84 (0.13)fbcde | 0.79 (0.13)facde | 0.69 (0.13)fabe | 0.69 (0.13)fabe | 0.63 (0.11)fabcd | <0.001 |
| Women (n = 1,106) | |||||||
| N | 86 | 88 | 179 | 316 | 342 | 87 | |
| SM, kg | 15.2 (2.3)acde | 14.1 (2.3)fcde | 14.5 (2.3)cde | 13.1 (2.3)fabde | 12.2 (2.3)fabce | 10.3 (2.5)fabcd | <0.001 |
| SM index, kg/m2 | 6.01 (0.82)de | 5.92 (0.86)e | 6.16 (0.91)cde | 5.89 (0.93)bde | 5.64 (0.93)fbce | 4.95 (1.04)fabcd | <0.001 |
| A50 MTH, cm | 4.82 (0.51)abcde | 4.25 (0.59)fcde | 4.22 (0.62)fcde | 3.78 (0.61)fabde | 3.52 (0.57)fabce | 3.17 (0.49)fabcd | <0.001 |
| P50 MTH, cm | 5.34 (0.57)abc | 5.60 (0.55)fe | 5.69 (0.57)fde | 5.62 (0.61)fe | 5.52 (0.68)be | 5.23 (0.80)abcd | <0.001 |
| A50:P50 MTH ratio | 0.91 (0.11)abcde | 0.77 (0.12)fcde | 0.75 (0.13)fcde | 0.68 (0.12)fabde | 0.64 (0.12)fabc | 0.62 (0.12)fabc | <0.001 |
Significance was set at p < 0.05
SM skeletal muscle mass, A50 MTH anterior 50 % thigh muscle thickness, P50 MTH posterior 50 % thigh muscle thickness
aSignificant group difference for ages 30–39
bSignificant group difference for ages 40–49
cSignificant group difference for ages 50–59
dSignificant group difference for ages 60–69
eSignificant group difference for ages 70–85
fSignificant group difference for ages 20–29
Relationships between SM index or A50/P50 MTH ratio and age
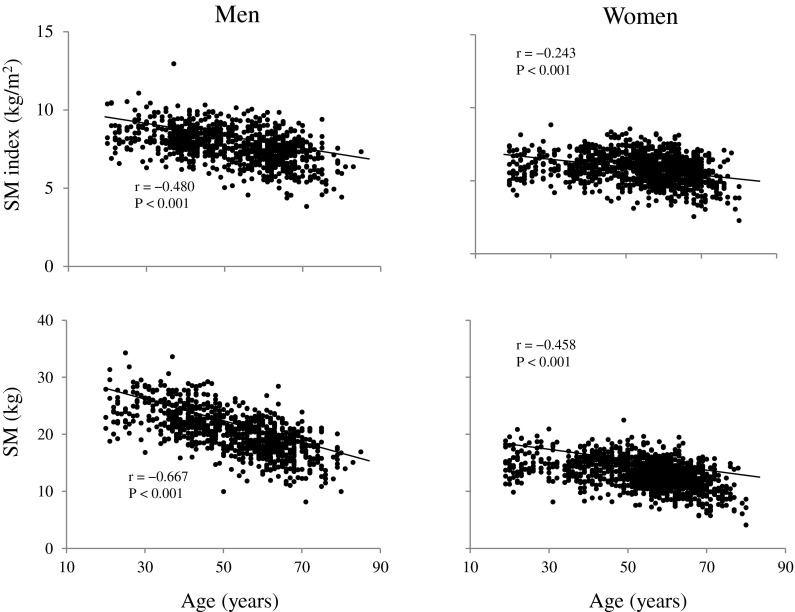
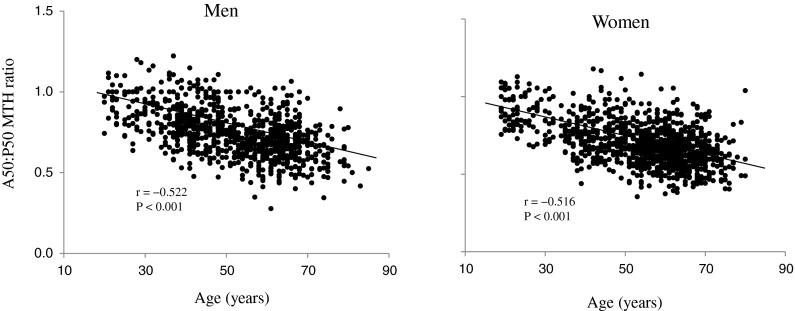
Age was inversely correlated with A50 MTH/thigh length in both men (r = −0.573, p < 0.001) and women (r = −0.487, p < 0.001). However, P50 MTH/thigh length was not correlated with age in men (r = −0.031) and women (r = 0.036). There were significant correlations between age and absolute SM as well as SM index in men (r = −0.667 and r = −0.480, both p < 0.001) and women (r = −0.458 and r = −0.243, both p < 0.001) (Fig. 1). Similarly, age was inversely correlated with A50/P50 MTH ratio in men (r = −0.522, p < 0.001) and women (r = −0.516, p < 0.001) (Fig. 2). A correlation coefficient between age and A50/P50 MTH was significantly higher (p > 0.001) than the correlation coefficient between age and SM index in women, but not in men.
Fig. 1.
Relationships between age and total skeletal muscle mass (SM) and SM index in men and women
Fig. 2.
Relationship between age and the anterior and posterior muscle thickness (A50:P50 MTH) ratio in men and women
Prevalence rates of sarcopenia detected by SM index
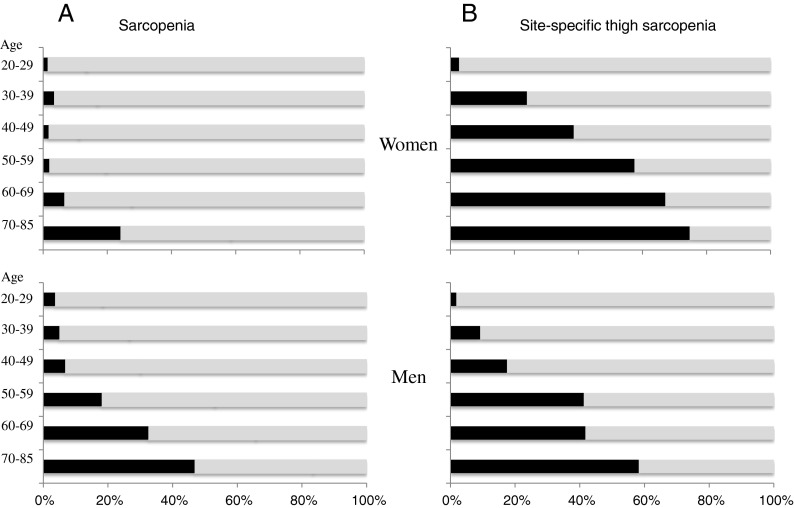
The prevalence of severe sarcopenia indicated an age-related increasing pattern in nonobese men, especially a marked increase after age 50. On the other hand, an age-related increasing pattern was not observed in nonobese women. The prevalence rate of sarcopenia was less than 3 % for women under the age of 60 (number of participants classified as severe sarcopenia: 1 in ages 20–29, 3 in ages 30–39, 3 in ages 40–49, and 6 in ages 50–59), 7 % for ages 60–69 (23 were classified), and 24 % for ages 70–85 (21 were classified). In men, the prevalence rate of sarcopenia was less than 7 % for those under the age of 50 (number of participants classified as severe sarcopenia: 2 in ages 20–29, 6 in ages 30–39, and 14 in ages 40–49), 18 % for ages 50–59 (32 were classified), 33 % for ages 60–69 (82 were classified), and 47 % for ages 70–85 (37 were classified) (Fig. 3).
Fig. 3.
The prevalence rates of severe sarcopenia detected by SM index and of site-specific thigh sarcopenia in men and women
Prevalence rates of site-specific thigh sarcopenia
The prevalence of site-specific thigh sarcopenia showed an age-related increasing pattern in both men and women. Number of participants classified as site-specific thigh sarcopenia was 1, 11, 36, 73, 105, and 46 in men (line up by age groups) and 2, 21, 69, 184, 232, and 65 in women (line up by age groups). The prevalence rate of site-specific thigh sarcopenia was 18 % for ages 40–49, approximately 40 % for ages 50–59 and 60–69, and 58 % for ages 70–85 in men and was 38 % for ages 40–49, 67 % for ages 60–69, and 75 % for ages 70–85 in women (Fig. 3).
Discussion
The main findings of the present study were that (1) the prevalence of sarcopenia detected by SM index indicated an age-related increasing pattern in men (especially >50 years), but not in women; (2) there was a high percentage of age-related decreases observed in the A50/P50 MTH ratio (an index of site-specific thigh sarcopenia) compared with the SM index; and (3) the prevalence of site-specific thigh sarcopenia displayed an age-related increasing pattern in both sexes, and it appears before it is able to be detected at the whole body level.
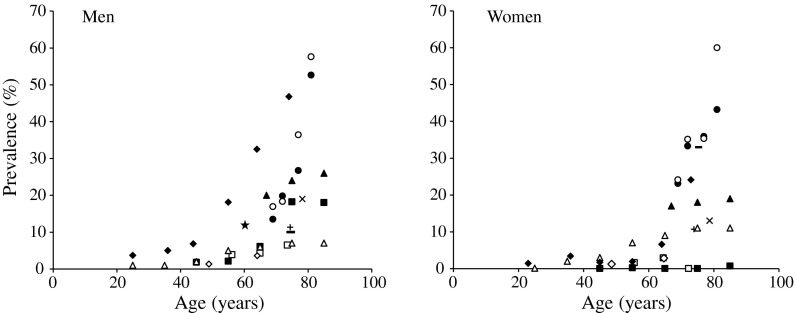
Based on definition of sarcopenia by Baumgartner et al. (aLM index by DXA), the prevalence of severe (class II) sarcopenia was more than 50 % for non-Hispanic and Hispanic men and approximately 60 % for Hispanic women aged 80 years or older (Baumgartner et al. 1998). Using the same diagnostic criteria, the prevalence of sarcopenia was approximately 4 % for European men under the age of 59 years, 4 % for those between 60 and 69 years, and 7 % for those over 70 years of age (Bijlsma et al. 2013). On the other hand, Janssen et al. (2002) reported a large-scale cross-sectional study using bioelectric impedance analysis and found that the prevalence of severe sarcopenia was less than 5 % for men and women aged 49 years or younger and approximately 10 % for men and women aged 70 years or older. In the present study, the prevalence rate of severe sarcopenia was relatively higher in nonobese men compared with those of the previous studies and was consistent with previous studies in women (Baumgartner et al. 1998; Bijlsma et al. 2013; Chien et al. 2008; Dufour et al. 2012; Janssen et al. 2002; Kim et al. 2012; Masanes et al. 2012; Rolland et al. 2003; Tanimoto et al. 2012; Tichet et al. 2008; Verschueren et al. 2012). Taken together with previous studies (Fig. 4), the results indicate that the prevalence of severe sarcopenia (2 SD below the sex-specific means of aLM index or SM index) is less than 7 % for men and 3 % for women under the age of 50 and a marked age-related increase in the prevalence of sarcopenia is observed after age 50 in both sexes.
Fig. 4.
Relationship between age and the prevalence of severe sarcopenia in men and women. When authors have presented age range only, we used median age of the age range, for example, 67.5 years for ages between 65 and 69. Plotting data: present study (filled diamond); non-Hispanic, Baumgartner et al. (1998) (filled circle); Hispanic, Baumgartner et al. (1998) (open circle); Bijlsma et al. (2013) (open square); Chien et al. (2008) (filled triangle), Dufour et al. (2012) (multiplication sign), Janssen et al. (2002) (open triangle), Kim et al. (2012) (filled square), Masanes et al. (2012) (minus sign), Tanimoto et al. (2012) (plus sign), Tichet et al. (2008) (open diamond), Verschueren et al. (2012) (filled star)
Our results showed that approximately 6 % of nonobese men and 2 % of women under the age of 50 were classified as having severe sarcopenia. A previous study reported that ~2 % of men and ~3 % of women under the age of 50 met the criteria for severe sarcopenia (Janssen et al. 2002). In another study, the prevalence rate of sarcopenia for subjects between the ages of 40–49 years was about 2 % in men and 0 % in women (Kim et al. 2012). The skeletal muscle mass maintained in later life is not only determined by the rate of muscle loss but also reflects the peak muscle mass attained in early life. Although the magnitude of physical disability as well as functional impairment may differ between severe sarcopenic young and older adults, a lower muscle mass obtained in youth and early adulthood may present a disadvantage for delaying or preventing sarcopenia.
In the present study, the prevalence of site-specific thigh sarcopenia was approximately 30 % of men and women between 30 and 39 years and 40 % of those between 40 and 49 years. Age-related changes in SM index were relatively constant under the age of 50 (Fig. 1), while the A50/P50 MTH ratio gradually decreased with age in both men and women (Fig. 2). Because posterior thigh (hamstrings) MTH was not significantly decreased among age groups under the age of 60 in men and under the age of 70 in women, the main reason for the age-related decrease in A50/P50 MTH ratio would be due to lower anterior thigh (quadriceps) MTH in older subjects (Table 2). A previous study reported that there were strong inverse correlations between age and anterior thigh MTH (r = −0.529 for men [n = 722] and r = −0.489 for women [n = 785]), but not to posterior thigh MTH (r = −0.068 for men and r = 0.167 for women) in both sexes (Abe et al. 2011b). Similar results were observed in the present study. Interestingly, the anterior and posterior thigh MTH ratio was inversely correlated to zigzag walking performance while it did not correlate to maximum walking performance (Abe et al. 2012c). Therefore, our results suggest that site-specific thigh sarcopenia may begin appearing before it can be detected at the whole body level and that this site-specific sarcopenia may be associated with a decrease in a relatively difficult task performance such as zigzag walking.
The reason for the site-specific decrease in muscle mass with age is largely unknown. Site-specific thigh sarcopenia is likely multifactorial as it is with the current dogmatic age-related model of homogeneous skeletal muscle mass loss. One possible factor may be the intensity and duration of physical activity completed over a lifetime. Due to changes in physical activity and/or age-related neuromuscular changes, it is conceivable that there may be a decline in anterior muscle activation with advancing age. This is supported by research that has observed site-specific losses in motor units with advancing age (Aagaard et al. 2010). In addition, age-related declines in androgen concentrations may also be playing some role in site-specific sarcopenia (Morley 2003). These decreases in basal levels of circulating hormones have also been implicated in other sarcopenic models (Mitchell et al. 2012). Hormone receptors are upregulated in exercising muscle, but not in nonexercising muscle. Due to possible site-specific reductions in muscle activation, it may be that there is also a decrease in hormonal binding in the anterior portion of the thigh (Morley 2003). Another player in site-specific sarcopenia may be insulin resistance. Insulin-resistant men have greater muscle mass loss with aging when compared to insulin-sensitive men (Lee et al. 2011), and there may be differential responses in different muscle groups. For example, type IIb fibers have been shown to have higher levels of insulin resistance (James et al. 1985). Lastly, the microstructure of certain muscles may also change with age as they do during disuse-induced atrophy. For example, disuse atrophy is always greater in antigravity muscles than in their antagonists (quadriceps vs. hamstrings) (Clark 2009). In addition, these age-related structural changes may cause the muscles in the anterior portion of the thigh to be held at a shortened length, which is related to a loss in the number of sarcomeres (Tardieu et al. 1980). Although the mechanisms are currently speculative, future work may be able to delineate the molecular mechanisms involved with site-specific sarcopenia.
In conclusion, our results demonstrated that the prevalence rate differs between sarcopenia detected by SM index and site-specific thigh sarcopenia. The site-specific muscular changes begin appearing before they are able to be detected by the SM index. Thus, we suggest that the anterior and posterior MTH ratio may be a novel marker to provide an earlier diagnosis (and treatment intervention) for the age-related loss of muscle mass.
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Abe T, Kondo M, Kawakami Y, Fukunaga T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol. 1994;6:161–170. doi: 10.1002/ajhb.1310060204. [DOI] [PubMed] [Google Scholar]
- Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days bed rest on muscle morphology. J Gravitat Physiol. 1997;4:S10–14. [PubMed] [Google Scholar]
- Abe T, Kawakami Y, Kondo M, Fukunaga T. Comparison of ultrasound-measured age-related, site-specific muscle loss between healthy Japanese and German men. Clin Physiol Funct Imaging. 2011;31:320–325. doi: 10.1111/j.1475-097X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- Abe T, Sakamaki M, Yasuda T, Bemben MG, Kondo M, Kawakami Y, Fukunaga T. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J Sports Sci Med. 2011;10:145–150. [PMC free article] [PubMed] [Google Scholar]
- Abe T, Bemben MG, Kondo M, Kawakami Y, Fukunaga T. Comparison of skeletal muscle mass to fat-free mass ratios among different ethnic groups. J Nutr Health Aging. 2012;16:534–538. doi: 10.1007/s12603-012-0015-2. [DOI] [PubMed] [Google Scholar]
- Abe T, Mitsukawa N, Thiebaud RS, Loenneke JP, Loftin M, Ogawa M. Lower body site-specific sarcopenia and accelerometer-determined moderate and vigorous physical activity: the HIREGASAKI study. Aging Clin Exp Res. 2012;24(6):657–62. doi: 10.3275/8758. [DOI] [PubMed] [Google Scholar]
- Abe T, Ogawa M, Loenneke JP, Thiebaud RS, Loftin M, Mitsukawa N. Relationship between site-specific loss of thigh muscle and gait performance in women: the HIREGASAKI study. Arch Gerontol Geriatr. 2012;55:e21–e25. doi: 10.1016/j.archger.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Abe T, Thiebaud RS, Loenneke JP, Loftin M, Bemben MG, Fukunaga T. Influence of severe sarcopenia on cardiovascular risk factors in nonobese men. Metab Syndr Relat Disord. 2012;10:407–412. doi: 10.1089/met.2012.0057. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross R, Garry PJ, Linderman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Belanger M, Townsend N, Foster C. Age-related differences in physical activity profiles of English adults. Prev Med. 2011;52:247–249. doi: 10.1016/j.ypmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Bijlsma AY, Meskers CGM, Ling CH, Narici M, Kurrle SE, Cameron ID, Westendrorp RG, Maier AB. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age. 2013;35(3):871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56:1710–1715. doi: 10.1111/j.1532-5415.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- Clark BC. In vivo alterations in skeletal muscle form and function after disuse atrophy. Med Sci Sports Exerc. 2009;41:1869–1875. doi: 10.1249/MSS.0b013e3181a645a6. [DOI] [PubMed] [Google Scholar]
- Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham study. J Gerontol A Biol Sci Med Sci. 2012;68(2):168–74. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, et al. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Ageing, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- James DE, Jenkins AB, Kraegen EW. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985;248:E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Getriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, Song G, Kim HJ, Choi YJ, Kim KM. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012;67:1107–1113. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59:1217–1224. doi: 10.1111/j.1532-5415.2011.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshits G, Malkin I, Williams FM, Hart DJ, Hakim A, Spector TD. Longitudinal study of variation in body mass index in middle-aged UK females. Age. 2012;34:1285–1294. doi: 10.1007/s11357-011-9299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masanes F, Culla A, Navarro-Gonzalez M, Navarro-Lopez M, Sacanella E, Torres B, et al. Prevalence of sarcopenia in healthy community-dwelling elderly in an urban area of Barcelona (Spain) J Nutr Health Aging. 2012;16:184–187. doi: 10.1007/s12603-011-0108-3. [DOI] [PubMed] [Google Scholar]
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. Hormones and the aging process. J Am Geriatr Soc. 2003;51:S333–S337. doi: 10.1046/j.1365-2389.2003.51344.x. [DOI] [PubMed] [Google Scholar]
- Newman AB, Kupelian V. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Yasuda T, Abe T. Component characteristics of thigh muscle volume in young and older healthy men. Clin Physiol Funct Imaging. 2012;32:89–93. doi: 10.1111/j.1475-097X.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- Park H, Park S, Shephard RJ, Aoyagi Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol. 2010;109:953–961. doi: 10.1007/s00421-010-1424-8. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–1124. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96:24–31. doi: 10.1007/s00421-005-0061-0. [DOI] [PubMed] [Google Scholar]
- Sawai S, Sanematsu H, Kanehisa H, Tsunoda N, Fukunaga T. Sexual-related difference in the level of muscular activity of trunk and lower limb during basic daily life actions. Jap J Phys Fit Sports Med. 2006;55:247–258. doi: 10.7600/jspfsm.55.247. [DOI] [Google Scholar]
- Shirasawa H, Kanehisa H, Kouzaki M, Masani K, Fukunaga T. Differences among lower leg muscles in long-term activity during ambulatory condition without any moderate to high intensity exercise. J Electromyogr Kinesiol. 2009;19:e50–e56. doi: 10.1016/j.jelekin.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity—definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto Y, Watanabe M, Sun M, Sugiura Y, Tsuda Y, Kimura M, Hayashida I, Kusabiraki T, Kono K. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch Gerontol Geriatr. 2012;55:e9–e13. doi: 10.1016/j.archger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Tardieu C, Tabary JC, Tardieu G, Tabary C. Adaptation of sarcomere numbers to the length imposed on the muscle. Adv Physiol Sci. 1980;24:99–114. [Google Scholar]
- Tichet J, Vol S, Goxe D, Salle A, Berrut G, Ritz P. Prevalence of sarcopenia in the French senior population. J Nutr Health Aging. 2008;12:202–206. doi: 10.1007/BF02982621. [DOI] [PubMed] [Google Scholar]
- Verschueren S, Gielen E, O’Neill TW, Pye SR, Adams JE, Ward KA, Wu FC, Szulc P, Laurent M, Claessens F, Vanderschueren D, Boonen S. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2012;24(1):87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- WHO (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO technical report series 894, Geneva, Switzerland [PubMed]