Abstract
Purpose
Antiseptics are powerful medical agents used for wound treatment and decontamination and have a high potential for defeating joint infections in septic surgery. Both chlorhexidine and polyhexanide are frequently used in clinical practice and have a broad antimicrobial range, but their effect on human osteoblasts has not been sufficiently studied. Our objective was to investigate the toxic effects of polyhexanide and chlorhexidine on human osteoblasts in vitro to evaluate their clinical applicability in septic surgery.
Methods
We isolated and cultivated human osteoblasts in vitro and assayed the toxic effects of chlorhexidine 0.1 % and polyhexanide 0.04 %, concentrations commonly applied in clinical practice. Toxicity analysis was performed by visualisation of cell structure, lactate dehydrogenase (LDH) activity and evaluation of vital cells. Toxicity was evaluated by microscopic inspection of cell morphology, trypan blue staining and determination of LDH release.
Results
Damaged cell structure could be shown by microscopy. Both antiseptics promoted LDH activity after incubation with osteoblasts. The evaluation of vital osteoblasts showed a significant decrease of vital cells.
Conclusions
Both antiseptics induced significant cell death of osteoblasts at optimum exposure. We therefore recommend cautious use of polyhexanide and chlorhexidine in septic surgery to avoid severe osteoblast toxicity.
Keywords: Human osteoblasts, in vitro, Polyhexanide, Chlorhexidine, Periprosthetic joint infections
Introduction
Periprosthetic joint infections are a common cause of severe complications in arthroplasty. Prosthetic joint replacement is now being performed more frequently than ever, and so its significant complications need to be reconsidered [1]. Despite modern surgical standards, periprosthetic joint infections arise in 0.4–2 % of primary elective arthroplasty and in over 5 % of revision implantation [2]. This causes not only significant morbidity and mortality but also an increasing financial burden on healthcare. The gold standard for infected joint replacement is a two-staged reimplantation [3, 4]. During this procedure mechanical elimination of bacteria through lavage and surgical debridement can be supported by antiseptic solutions. However, all of the commonly used antiseptic irrigants exhibit considerable tissue toxicity, as antiseptics do not discriminate between eukaryotic and prokaryotic cells [5–7]. Nevertheless, local treatment with antiseptics is still a standard procedure in septic arthroplasty.
One of the most frequently used antiseptics in orthopaedic surgery is polyhexanide [8–10]. Literature reviews have revealed very limited data about the effects of polyhexanide exposure to human osteoblasts. In previous studies, we were able to show a superiority of polyhexanide compared with other antiseptics in treating human chondrocytes [11–13]. Polyhexanide has a late antiseptic onset of five to 20 minutes and therefore requires prolonged exposure time [14]. This can be disadvantageous in surgical practice, as it extends the medical intervention whereas limiting application time reduces the antiseptic effect.
However, the alternative use of chlorhexidine in orthopaedic surgery has not been studied sufficiently. Chlorhexidine is a powerful antiseptic agent that has successfully been used in endodontics and periodontology at standard concentrations of 0.1 % and 2 %. Chlorhexidine (2–4 %) can be applied for surgical-site and skin preparation, coating of central venous catheters and for hand disinfection [15]. Significant continuity by the slow release from hard or soft oral tissues has also been observed ensuring prolonged antimicrobial activity following application [16–19]. In contrast to polyhexanide, povidone iodide, hydrogen peroxide and chlorhexidine do not lose full antiseptic activity in the presence of blood or other liquids [15, 20]. Furthermore, treatment with 0.05 % chlorhexidine has no negative effects on wound healing [21, 22]. When used in lavage, chlorhexidine 0.05 % eliminates 99.8 % of contaminating bacteria within one minute in a tissue model [20]. Recent studies show that non-osteoarthritic cartilage treated with chlorhexidine 0.05 % followed by extensive rinsing was not significantly affected [23].
Interestingly, the reason for the limited use of chlorhexidine in orthopaedic surgery is rarely documented in the literature. Different case reports show toxic effects of chlorhexidine on human cartilage after open or arthroscopic surgery. In all cases, unspecified or high concentrations of chlorhexidine were used for an undefined time of treatment [24–27]. The impact of chlorhexidine on primary human osteoblasts is still unclear. We therefore compared effects of polyhexanide with regularly used low-concentrate (0.1 %) chlorhexidine on human osteoblasts. We hypothesised that treatment with low concentrations of chlorhexidine has toxic effects on human osteoblasts equal to those of polyhexanide.
Materials and methods
Tissue culture plasticware from TPP (Trasadingen, Switzerland) was used. Culture medium, phosphate-buffered saline (PBS), rypsin and foetal calf serum (FCS) were from Biochrom (Berlin, Germany). Other reagents were purchased from Sigma-Aldrich (Deisenhofen, Germany) or are otherwise specified below.
Osteoblast isolation, culture and treatment
Bone material was obtained from four donors with knee osteoarthritis treated by total knee arthroplasty. Prior to extraction, the donors showed no signs of infection in clinical examination or blood values. Experimental protocols were approved by the local ethics committee. Bone fragments were separated from cartilage and manually minced to fragments sized one to four cubic millimetres. The next step consisted of washing bone fragments twice with PBS. Then they were suspended in osteogenic medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) Ham’s F12 with 10 % FCS, 1 % L-glutamine, 1 % penicillin/streptomycin and 1 % vitamin solution, and cultured at 37 °C, 95 % air and 5 % CO2. After osteoblast subconfluency was reached, experiments were performed. Human osteoblasts were either treated with 0.04 % polyhexanide (Serag Wiessner, Naila, Germany) or 0.1 % chlorhexidine freshly prepared from 20 % chlorhexidine digluconate (Sigma Aldrich, Deisenhofen, Germany) and Aqua ad injectabilia (B. Braun Melsungen AG, Melsungen, Germany).
Determination and analysis of cell morphology: light microscopy and trypan blue staining
Human osteoblasts were cultured in 24-well plates and treated with 300 μl of polyhexanide 0.04 % or chlorhexidine 0.1 % for one, five and ten minutes and immediately washed with PBS. PBS-treated cells were used as a negative control and 2 % Triton X 100 as a positive control. Immediately after treatment, osteoblasts were evaluated by phase-contrast light microscopy (Axiovert 40 C Light Microscope, lens 10 × 0.25, ocular 10 × 18 Zeiss, Gottingen, Germany) and photographed using a digital single-lens reflex camera (Canon EOS 550D, 18 Megapixels, Japan). Digital photos were equalised using Adobe Photoshop Elements 9. For improved visualisation of cell morphology and viability, cells were stained with 300 μl trypan blue 0.4 % immediately after incubation with antiseptics for one and ten minutes. Trypan blue cannot pass intact cell membranes and therefore only stains defective cells whereas vital cells remain unaffected. Results were assessed by light microscopy, and digital photos were taken.
Cell lysis: lactate dehydrogenase enzyme activity
Twenty-four-well plates containing subconfluent osteoblasts were challenged with polyhexanide 0.04 % or chlorhexidine 0.1 %, PBS (negative control) and Triton X 100 1 % (positive control) for one, five and ten minutes. LDH activity in the supernatant was determined by the photometric measurement (OD 490 nm) of the reduction of sodium pyruvate in the presence of nicotinamide adenine dinucleotide, reduced (NADH), and expressed as percentage of total enzyme activity liberated from osteoblasts in the presence of the antiseptic. For this purpose, we used the LDH Cytotoxicity Detection Kit (Roche, Germany).
Vital cell counting: CASY cell counter
CASY cell counting technology was used to evaluate vital cell counts after antiseptic treatment. Osteoblasts were seeded in 24-well plates at a density of 2 × 104 cells per well overnight. For antiseptic treatment, polyhexanide 0.4 % and chlorhexidine 0.1 % were used, as well as PBS (negative control) and 2 % Triton X 100 (positive control) for one, five and ten minutes. After being washed with PBS, cells were detached with 100 μl trypsin. Living cell numbers were determined using the CASY Cell Counter and Analyser System (CASY®1 TTC Cell Counter, Schärfe System, Reutlingen, Germany).
Statistical analysis
A nonparametric Wilcoxon matched-pairs test was used for statistical significance of LDH activity; CASY results were evaluated by comparison of multiple values according to Bonferroni test. A p value of less than 0.05 was considered significant, and under 0.001 as highly significant. Statistical analysis was performed with IBM SPSS Statistics 21 software, and Microsoft Office Excel 2007 for creating graphics.
Results
Light microscopy
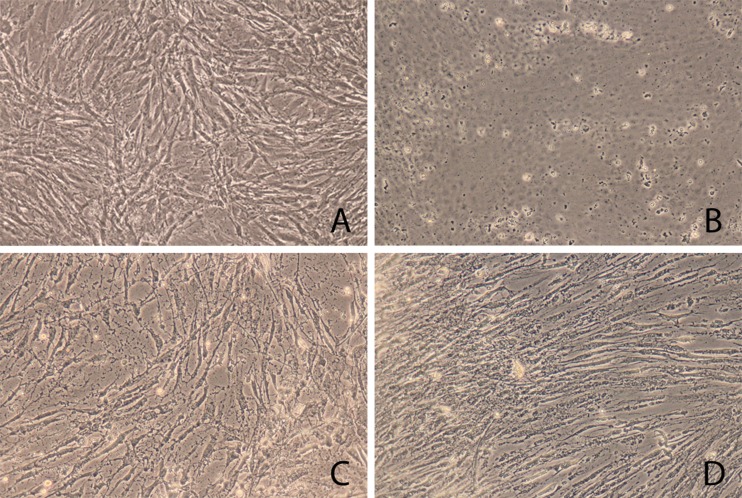
Light microscopy revealed increased cell defects and structural impairment of human osteoblasts incubated with polyhexanide and chlorhexidine compared with cells without antiseptic treatment, as shown in Fig. 1. Osteoblasts treated with PBS showed a regular cell structure and morphology at subconfluency with intercellular contacts. Cell cultures treated with antiseptics showed a darker and non homogeneous cytoplasm.
Fig. 1.
Light microscopy. Damage to osteoblasts after 5 min of antiseptic incubation as revealed by light microscopy. a Control osteoblasts treated with phosphate-buffered saline (PBS). b Osteoblasts treated with Triton X 100, positive control. c Chlorhexidine 0.1 %. d Polyhexanide 0.04 %
Chlorhexidine rapidly caused a change in osteoblast morphology. We noted a non homogeneous range of both shrunken cells with membrane-bound bodies and enlarged osteoblasts with inconsistent cell borders, as well as a moderate number of detached cells. Reduced intercellular contact and consecutively undefined cell borders were also visible. Polyhexanide incubation showed a consecutive shrinking, cell membrane blebbing and reduction of intercellular adhesion. Triton, known to be a mediator of cell necrosis, induced loss of adhesion, shrinking and loss of cell structure in all osteoblasts and served as a positive control.
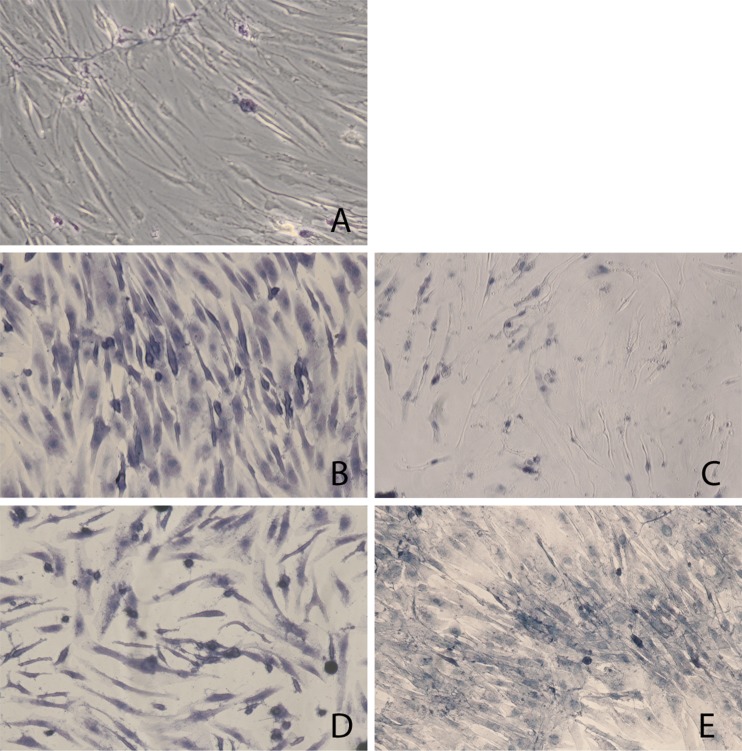
For an enhanced microscopic interpretation of cell viability, we used trypan blue staining, as shown in Fig. 2. The majority of control osteoblast cultures with PBS showed no trypan blue uptake. However, chlorhexidine treatment caused staining in most osteoblasts within one minute. Cell staining did not increase after ten minutes incubation with chlorhexidine. In comparison, polyhexanide-treated cells were less affected after one minute and contained both blue-stained and nonstained cells. Trypan blue uptake increased after ten minutes of polyhexanide treatment, as the majority of cells were damaged.
Fig. 2.
Trypan blue. Trypan blue staining of defective osteoblasts after antiseptic treatment for 1 and 10 min. a Untreated osteoblasts (control). b Chlorhexidine 1 min. c Polyhexanide 1 min. d Chlorhexidine 10 min. e Polyhexanide 10 min
LDH
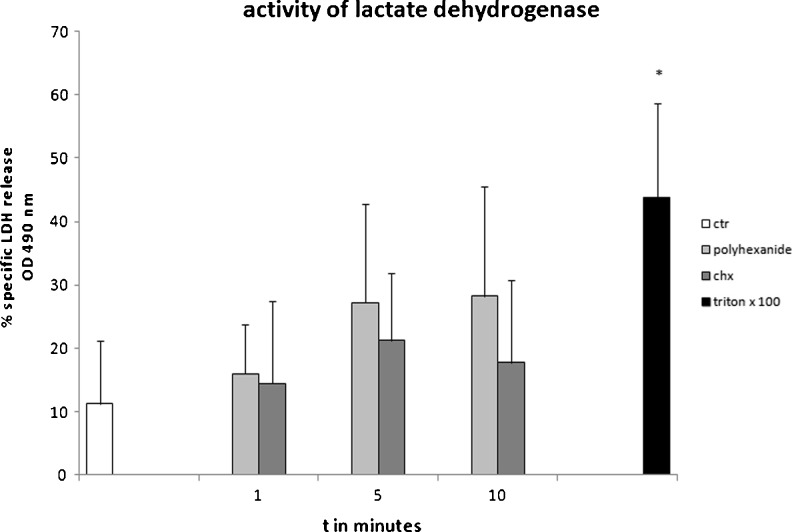
In the presence of antiseptics, LDH activity increased compared with control osteoblast supernatant, as shown in Fig. 3. However, the measured elevation was not significant.
Fig. 3.
Lactate dehydrogenase (LDH) activity in the supernatant of osteoblasts treated with antiseptics. Osteoblasts were incubated with chlorhexidine 0.1 % and polyhexanide 0.04 % for 1, 5 and 10 min following enzyme-linked immunosorbent assay (ELISA) analysis of LDH activity in the supernatant, n = 4. Values given ± standard error of mean (SEM). Nonparametric Wilcoxon matched-pairs test. Average of four independently performed experiments. *p < 0.05
Overall, osteoblasts exposed to polyhexanide showed higher levels and an increasing release of LDH over time, peaking at 28 % specific LDH release after ten minutes incubation time. In contrast, maximum specific LDH release for chlorhexidine-incubated osteoblasts was 21 % after five minutes, and a following decrease was measured after ten minutes of incubation time. Triton X 100 as an acknowledged inducer of cell lysis revealed a significant increase of LDH in comparison with the control.
CASY cell counter
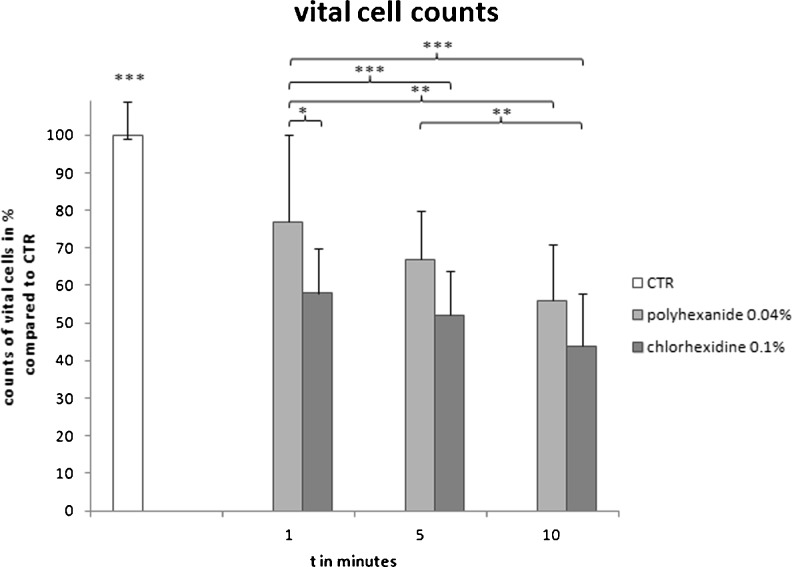
Cell viability was further examined by CASY cell counting technology, as shown in Fig. 4. Incubation of osteoblasts with antiseptics resulted in a highly significant reduction of vital cell counts from one minute onwards (p < 0.001). Overall, osteoblasts treated with polyhexanide showed higher vital cell count rates than those incubated with chlorhexidine. Vital cell counts were significantly higher in osteoblasts incubated with polyhexanide for one minute (77 % vs control) compared with osteoblasts treated with chlorhexidine for one minute (58 %, p < 0.05), five minutes (52 %, p < 0.001) and ten minutes (44 %, p < 0.001). However, polyhexanide incubation for five minutes (67 %) and ten minutes (56 %) did not differ significantly from one-minute chlorhexidine treatment (58 %).
Fig. 4.
CASY cell counting. Vital cell counts after antiseptic treatment in per cent of control vital count. Untreated osteoblasts are shown compared with osteoblasts treated with chlorhexidine or polyhexanide for 1, 5 and 10 min. 100 % = mean of vital cells in control (CTR) = 17.505 cells. Values given as mean ± standard error of mean (SEM). Comparison of multiple variables according to Bonferroni test. *p < 0.05, **p < 0.01, ***p < 0.001
Vital cell counts under polyhexanide incubation were significantly reduced over time; one minute of treatment showed 77 % vital cells compared with control (CTR), whereas ten minutes of incubation reduced vital cells to 56 % (p < 0.01). In contrast, vital cell counts following chlorhexidine treatment were initially lower (58 %) but showed no further significant time-dependent reduction. Least vital cell counts were detected in osteoblasts incubated with chlorhexidine for ten minutes (44 %), but these were significantly less than polyhexanide-treated cells incubated for five minutes (67 %, p < 0.01).
Discussion
We investigated the toxic effects of polyhexanide, the most frequently used antiseptic in orthopaedic surgery, and chlorhexidine, an agent commonly used in the oral cavity, on human bone cells using concentrations commonly applied in clinical practice. We have shown that antiseptic treatment leads to morphological changes in human osteoblasts as observed by light microscopy. Trypan blue staining revealed that chlorhexidine led to instant cell damage in most osteoblasts, whereas polyhexanide showed a time-dependent impact. A significant reduction of vital cells for both antiseptics was seen. LDH assay revealed an increase of LDH in the supernatant of osteoblasts incubated with both antiseptics, indicating a minor degree of advanced cell death in terms of necrosis. Conversely, measured LDH activity did not prove significant compared with the control. However, protein precipitation has previously been described for cationic antiseptics such as chlorhexidine and polyhexanide [28, 29], which could possibly affect the LDH enzyme. This could therefore lead to an overall reduction in LDH enzyme activity in the supernatant of osteoblasts, resulting in an underestimation of LDH release in cells treated with antiseptics.
Chlorhexidine treatment caused morphological signs of cell death. Shrunken cells with a blebbing cell membrane most likely indicate apoptosis, whereas enlarged osteoblasts with inconsistent cell borders suggest necrosis [30, 31]. Both types of cell death under chlorhexidine incubation have been previously described on the osteoblastic Saos-2 cell line by Giannelli et al. [32]. In our study, chlorhexidine was found to damage human osteoblasts from an incubation time of one minute onwards in terms of cell morphology, vitality and count. Similar immediate toxic influences have been reported on alveolar bone cells [33] and osteoblastic cell lines [32, 34]. No significant time dependency could be found in our experiments for exposure times of one to ten minutes. Exposure times of up to 20 minutes have been studied describing increasing cytotoxicity over time [35]. Giannelli et al. Cabral et al. Lee et al. and Verdugo et al. showed a dose-dependent cytotoxic effect of chlorhexidine on osteoblasts [32–34, 36]. It might therefore be useful for use in intraoperative procedures as long as lower concentrations and quick elimination are guaranteed. However, the value of chlorhexidine described for oral cavity treatment may complicate the definition of the effective application time.
Polyhexanide incubation induced shrunken, budded cells with globular formations showing less intercellular contact. These morphological changes in human osteoblasts most likely indicate apoptosis [30, 31]. We previously showed an early apoptotic effect of polyhexanide on human chondrocytes [11]. Results of the study reported here reveal a highly significant time-dependent impairment of osteoblasts from an incubation time of one minute onwards. Polyhexanide treatment was less cytotoxic after one, five and ten minutes of incubation time in terms of cell count and viability compared with chlorhexidine. However, it is important to consider the required exposure times for ensuring antiseptic efficacy of both agents for clinical comparability. Light microscopy with trypan blue staining revealed that ten minutes of polyhexanide incubation and one minute of chlorhexidine treatment led to a comparable amount of impaired cells. CASY cell counting indicates that cells are as equally damaged after a five and ten incubation with polyhexanide as after a one minute of treatment with chlorhexidine. It can therefore be assumed that on reaching its full antiseptic power after five to 20 minutes [14], polyhexanide is just as cytotoxic as chlorhexidine, which takes effect within the first minute [20].
In our study, we used human osteoblasts to obtain results that match in vivo conditions as closely as possible. However, comparability can only be achieved to a limited extent. Isolated human tissue is always subject to donor-related individual variations regarding age, gender and hormone levels [37]. Additionally, the in vitro osteoblast cell culture does not offer all functions of a human bone tissue. Osteoblasts are exposed directly to the agent rather than passing different barriers of cell tissue in vivo. Also, antiseptic dilution through accumulating tissue fluid, regulation of body temperature, influences on immunological effects and damage resulting from the joint infection itself are not considered during in vitro analysis. Additional investigations are therefore needed to evaluate the full extent of antiseptic effects on human bone tissue.
Conclusion
Results of this study indicate that both polyhexanide 0.04 % and chlorhexidine 0.1 % have a cytotoxic effect on human osteoblasts in vitro. At their individual optimum exposures, polyhexanide and chlorhexidine are equally cytotoxic. We conclude that due to the severe osteoblast toxicity, both antiseptics should only be used for septic surgery after careful consideration. However, both polyhexanide and chlorhexidine can be useful for intraoperative procedures if their antiseptic potential, concentration and exposure time are carefully considered. Further investigations on aspects of dose dependency and in vivo conditions are required.
Acknowledgments
Conflict of interest
None.
References
- 1.Bernard L, Hoffmeyer P, Assal M, et al. Trends in the treatment of orthopaedic prosthetic infections. J Antimicrob Chemother. 2004;53:127–129. doi: 10.1093/jac/dkh033. [DOI] [PubMed] [Google Scholar]
- 2.Gollwitzer H, Diehl P, Gerdesmeyer L, Mittelmeier W. [Diagnostic strategies in cases of suspected periprosthetic infection of the knee. A review of the literature and current recommendations] Orthop. 2006;35:904. doi: 10.1007/s00132-006-0977-z. [DOI] [PubMed] [Google Scholar]
- 3.Jämsen E, Stogiannidis I, Malmivaara A, et al. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macheras GA, Koutsostathis SD, Kateros K, et al. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int J Clin Exp Res Hip Pathol Ther. 2012;22(Suppl 8):S54–61. doi: 10.5301/HIP.2012.9571. [DOI] [PubMed] [Google Scholar]
- 5.Atiyeh BS, Dibo SA, Hayek SN. Wound cleansing, topical antiseptics and wound healing. Int Wound J. 2009;6:420–430. doi: 10.1111/j.1742-481X.2009.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds. 2003;15:149–166. [Google Scholar]
- 7.Hirsch T, Koerber A, Jacobsen F, et al. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J Surg Res. 2010;164:344–350. doi: 10.1016/j.jss.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Ince A, Schütze N, Hendrich C, et al. Effect of polyhexanide and gentamycin on human osteoblasts and endothelial cells. Swiss Med Wkly. 2007;137:139–145. doi: 10.4414/smw.2007.11434. [DOI] [PubMed] [Google Scholar]
- 9.Kock H-J, Ernst D, Jethon F, Fabry W. In-vitro analysis of the effect of gentamicin and polyhexanide on bone tissue. Int Orthop. 2013;37:761–767. doi: 10.1007/s00264-013-1786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Röhner E, Hoff P, Pfitzner T, et al. Limited use of antiseptics in septic surgery. J Investig Surg Off J Acad Surg Res. 2012;25:311–316. doi: 10.3109/08941939.2011.648718. [DOI] [PubMed] [Google Scholar]
- 11.Röhner E, Seeger JB, Hoff P, et al. Preferred use of polyhexanide in orthopedic surgery. Orthopedics. 2011;34:e664–668. doi: 10.3928/01477447-20110826-10. [DOI] [PubMed] [Google Scholar]
- 12.Röhner E, Kolar P, Seeger JB, et al. Toxicity of antiseptics against chondrocytes: What is best for the cartilage in septic joint surgery? Int Orthop. 2011;35:1719–1723. doi: 10.1007/s00264-010-1178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumburger J, Beckmann J, Springorum H-R, et al. Toxicity of antiseptics on chondrocytes in vitro. Z Für Orthop Unfallchirurgie. 2010;148:39–43. doi: 10.1055/s-0029-1186127. [DOI] [PubMed] [Google Scholar]
- 14.Kramer A, Daeschlein G, Kammerlander G, et al. Konsensusempfehlung zur Auswahl von Wirkstoffen für die Wundantiseptik. Hyg Med. 2004;5:147–157. [Google Scholar]
- 15.Lim K-S, Kam PCA. Chlorhexidine–pharmacology and clinical applications. Anaesth Intensive Care. 2008;36:502–512. doi: 10.1177/0310057X0803600404. [DOI] [PubMed] [Google Scholar]
- 16.Cecchin D, de Almeida JFA, Gomes BPFA, et al. Effect of chlorhexidine and ethanol on the durability of the adhesion of the fiber post relined with resin composite to the root canal. J Endod. 2011;37:678–683. doi: 10.1016/j.joen.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Hitz Lindenmüller I, Lambrecht JT. Oral care. Curr Probl Dermatol. 2011;40:107–115. doi: 10.1159/000321060. [DOI] [PubMed] [Google Scholar]
- 18.Matthews D. No difference between 0.12% and 0.2% chlorhexidine mouthrinse on reduction of gingivitis. Evid Based Dent. 2011;12:8–9. doi: 10.1038/sj.ebd.6400771. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Stojicic S, Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod. 2011;37:657–661. doi: 10.1016/j.joen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Taylor GJ, Leeming JP, Bannister GC. Effect of antiseptics, ultraviolet light and lavage on airborne bacteria in a model wound. J Bone Joint Surg Br. 1993;75:724–730. doi: 10.1302/0301-620X.75B5.8376427. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez IR, Swaim SF, Nusbaum KE, et al. Effects of chlorhexidine diacetate and povidone-iodine on wound healing in dogs. Vet Surg Vs. 1988;17:291–295. doi: 10.1111/j.1532-950X.1988.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 22.Severyns AM, Lejeune A, Rocoux G, Lejeune G. Non-toxic antiseptic irrigation with chlorhexidine in experimental revascularization in the rat. J Hosp Infect. 1991;17:197–206. doi: 10.1016/0195-6701(91)90231-V. [DOI] [PubMed] [Google Scholar]
- 23.Best AJ, Nixon MF, Taylor GJS. Brief exposure of 0.05% chlorhexidine does not impair non-osteoarthritic human cartilage metabolism. J Hosp Infect. 2007;67:67–71. doi: 10.1016/j.jhin.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Bellen P. Chondrolysis caused by chlorhexidine. Acta Orthop Belg. 1987;53:112–113. [PubMed] [Google Scholar]
- 25.Douw CM, Bulstra SK, Vandenbroucke J, et al. Clinical and pathological changes in the knee after accidental chlorhexidine irrigation during arthroscopy. Case reports and review of the literature. J Bone Joint Surg Br. 1998;80:437–440. doi: 10.1302/0301-620X.80B3.8519. [DOI] [PubMed] [Google Scholar]
- 26.Rombouts JJ, Wery PE, Delloye C, et al. Proper use of irrigation solutions in orthopedic surgery. Apropos of a case of chondrolysis due to chlorhexidine] Acta Orthop Belg. 1986;52:685. [PubMed] [Google Scholar]
- 27.Tricoit M, Sillion D, Yaffi D, et al. Chondrolyse á la chlorhexidine: un cas contrôlé histologiquement. Rev Chir Orthop. 1984;70:348–9. [Google Scholar]
- 28.Ikeda T, Ledwith A, Bamford CH, Hann RA. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim Biophys Acta. 1984;769:57–66. doi: 10.1016/0005-2736(84)90009-9. [DOI] [PubMed] [Google Scholar]
- 29.Wiegand C, Abel M, Kramer A, et al. Proliferationsförderung und Biokompatibilität von Polihexanid. Gms Krankenhaushyg Interdiszip. 2007;2:2007–2. [Google Scholar]
- 30.Walker NI, Harmon BV, Gobé GC, Kerr JF. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- 31.Martin D, Lenardo M (2001) Morphological, biochemical, and flow cytometric assays of apoptosis. Curr Protoc Mol Biol doi: 10.1002/0471142727.mb1413s49 [DOI] [PubMed]
- 32.Giannelli M, Chellini F, Margheri M, et al. Effect of chlorhexidine digluconate on different cell types: a molecular and ultrastructural investigation. Toxicol Vitro Int J Publ Assoc Bibra. 2008;22:308–317. doi: 10.1016/j.tiv.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Cabral CT, Fernandes MH. In vitro comparison of chlorhexidine and povidone-iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin Oral Investig. 2007;11:155–164. doi: 10.1007/s00784-006-0094-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee T-H, Hu C-C, Lee S-S, et al. Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int Endod J. 2010;43:430–435. doi: 10.1111/j.1365-2591.2010.01700.x. [DOI] [PubMed] [Google Scholar]
- 35.Bhandari M, Adili A, Schemitsch EH. The efficacy of low-pressure lavage with different irrigating solutions to remove adherent bacteria from bone. J Bone Joint Surg Am. 2001;83-A:412–419. doi: 10.2106/00004623-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Verdugo F, Sáez-Rosón A, Uribarri A, et al. Bone microbial decontamination agents in osseous grafting: an in vitro study with fresh human explants. J Periodontol. 2011;82:863–871. doi: 10.1902/jop.2010.100514. [DOI] [PubMed] [Google Scholar]
- 37.Czekanska EM, Stoddart MJ, Richards RG, Hayes JS. In search of an osteoblast cell model for in vitro research. Eur Cell Mater. 2012;24:1–17. doi: 10.22203/ecm.v024a01. [DOI] [PubMed] [Google Scholar]