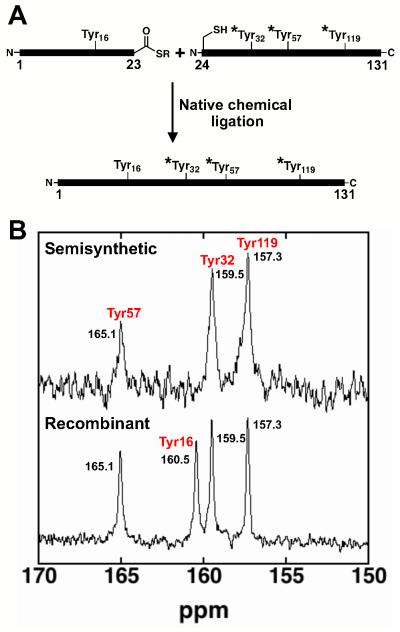
Figure 1.
13C-NMR spectra of site-specifically labeled semi-synthetic R15K/D21N/D24C/D40N KSI to assign the identity of the ionizing Tyr residue. (A) A synthetic fragment (1-23) bearing unlabeled Tyr16 is ligated to a recombinant fragment (24-131) bearing 13Cζ-Tyr at residues 32, 57, and 119 (*Tyr). (B) The 13C-NMR spectrum for fully recombinant R15K/D21N/D24C/D40N KSI with 13Cζ-Tyr residues and semi-synthetic R15K/D21N/D24C/D40N KSI with unlabeled Tyr16. The spectrum of semi-synthetic KSI lacks the peak at 160.5 ppm allowing assignment of this peak to Tyr16. The peak at 157.3 ppm was previously assigned to Tyr119, and the peak at 165.1 was attributed to Tyr57 as described in the text.