Figure 6.
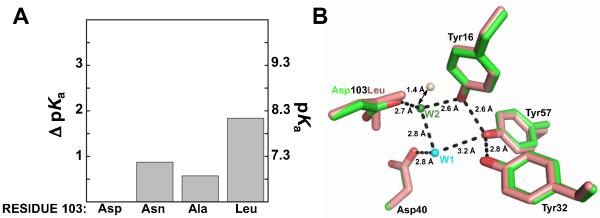
Effects of Asp103 mutations on the Tyr pKa. (A) The ΔpKa values are relative to the pKa value of Asp40Asn. Values are from Table 2. (B) Superposition of structural models of Met116Ala (green, PDB ID 3RGR) and Asp103Leu (salmon, PDB ID 1W00) suggests the position of an organized water molecule in the oxyanion hole is perturbed in Asp103Leu (salmon sphere) compared to wild type (green sphere). Wat1 is not observed in the Asp103Leu structure.
