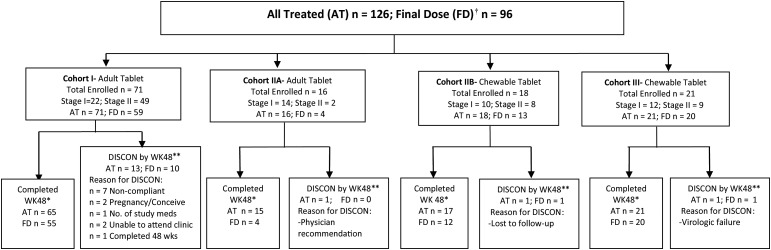
Figure 1.
Overall disposition of patients. Patients were enrolled in only 1 cohort. †Received only the final recommended dose of raltegravir. *Patient was on study drug to at least Study Day 295. **Disposition is provided for all treated patients based on patient status at the week 48 study visit. In some instances, patients discontinued after Study Day 295 but prior to or at the week 48 study visit and so the number of patients discontinued by week 48 is higher than expected based on the number completed at week 48.