Abstract
Background and Aims
Plant–pollinator interactions are thought to have shaped much of floral evolution. Yet the relative importance of pollinator shifts and coevolutionary interactions for among-population variation in floral traits in animal-pollinated species is poorly known. This study examined the adaptive significance of spur length in the moth-pollinated orchid Platanthera bifolia.
Methods
Geographical variation in the length of the floral spur of P. bifolia was documented in relation to variation in the pollinator fauna across Scandinavia, and a reciprocal translocation experiment was conducted in south-east Sweden between a long-spurred woodland population and a short-spurred grassland population.
Key Results
Spur length and pollinator fauna varied among regions and habitats, and spur length was positively correlated with the proboscis length of local pollinators. In the reciprocal translocation experiment, long-spurred woodland plants had higher pollination success than short-spurred grassland plants at the woodland site, while no significant difference was observed at the grassland site.
Conclusions
The results are consistent with the hypothesis that optimal floral phenotype varies with the morphology of the local pollinators, and that the evolution of spur length in P. bifolia has been largely driven by pollinator shifts.
Keywords: Ecotype, evolution of floral traits, moth pollination, spur length, Platanthera bifolia, pollination ecotypes, pollination success, pollinator shift, population differentiation, proboscis length, speciation
INTRODUCTION
Pollinator-driven divergent selection on floral traits has been implied as an important mechanism of angiosperm diversification and speciation (Dodd et al., 1999; Kay and Sargent, 2009; Vamosi and Vamosi, 2010; Van der Niet and Johnson, 2012). Population differentiation in floral traits of animal-pollinated plants may be the result of adaptive evolution driven by selection exerted by pollinators, but may also be influenced by selection exerted by antagonists and abiotic factors, and by random processes (Nuismer et al., 2000; Fenster et al., 2004; Strauss and Irwin, 2004; Herrera et al., 2006; Rausher 2008; Gómez et al., 2009; Anderson et al., 2010a; Armbruster et al., 2010). The hypothesis that the evolution of floral traits is driven by pollinator-mediated selection has been examined at the interspecific level by phylogenetic studies mapping shifts in pollinators and floral characters (e.g. Armbruster, 1993; Armbruster and Baldwin, 1998; Johnson et al., 1998; Wilson et al., 2004; Whittall and Hodges, 2007; Van der Niet and Johnson, 2012). At the intraspecific level, the hypothesis can be explored by examining the correlation between the morphology of the locally most important pollinator and floral morphology (Brink, 1980; Robertson and Wyatt, 1990; Johnson and Steiner, 1997; Boyd, 2002). Studies of species radiations within the genera Aquilegia (Whittall and Hodges, 2007), Disa (Johnson et al., 1998), Dalechampia (Armbruster, 1993; Armbruster and Baldwin, 1998), Penstemon (Castellanos et al., 2003; Wilson et al., 2004), Mimulus (Bradshaw and Schemske, 2003) and Iochroma (Smith et al., 2008; Fenster et al., 2009) support the hypothesis that shifts to new pollinators are associated with characteristic changes in floral traits. Less is known about the extent to which floral variation within species is the product of selection mediated by pollinators (Herrera et al., 2006; Anderson and Johnson, 2008; Gómez et al., 2009; Pauw et al., 2009; Peter and Johnson, 2014).
Reciprocal transplant experiments provide a critical test of the hypothesis that populations have adapted to local environmental conditions (Clausen et al., 1940; Kawecki and Ebert, 2004; Ågren and Schemske, 2012), and have demonstrated adaptive differentiation among populations of a large number of both plant and animal species (Leimu and Fisher, 2008; Hereford, 2009). To test the idea that plant populations are adapted to the local pollination environment requires reciprocal transplant experiments in which pollination success is evaluated and the relationship between pollination success and plant fitness established. However, few translocation experiments have been conducted to test the hypothesis of intraspecific adaptive divergence in relation to the composition or behaviour of local pollinators (e.g. Newman et al., 2012; Sun et al., 2014).
Both coevolutionary interactions and pollinator shifts may contribute to pollinator-driven evolution of floral traits. The coevolution hypothesis suggests that both plant and pollinator traits evolve as a result of reciprocal selection (Janzen, 1980; Anderson and Johnson, 2008; Harder and Johnson, 2009; Pauw et al., 2009; Zhang et al., 2013). In contrast, the pollinator-shift hypothesis suggests that floral evolution is driven by pollinator-mediated selection, but with no reciprocal evolution of the pollinator (Johnson and Steiner, 1997; Wasserthal, 1997; Whittall and Hodges, 2007; Anderson and Johnson, 2008). Trait covariation among populations of a plant and its primary pollinator would be consistent with coevolution (but see Anderson et al., 2010b; Nuismer et al., 2010). Alternatively, in a situation where the dominating pollen vector varies among habitats, a correlation between plant traits and the characteristics of the dominating pollinator would be consistent with the hypothesis of floral evolution by pollinator shifts. Coevolutionary interactions and pollinator shifts have been proposed as alternative hypotheses (e.g. Whittall and Hodges, 2007), but both processes are likely to contribute to floral evolution in any given system, and the challenge is rather to determine their relative importance.
In animal-pollinated plants, the lengths of corolla tubes and floral spurs are expected to affect the mechanical fit to pollinators and thereby pollination efficiency (Darwin, 1862; Nilsson, 1988; Johnson and Steiner, 2007; Boberg and Ågren, 2009; Muchhala and Thomson, 2009; Sletvold and Ågren, 2011). The evolution of long floral spurs has been attributed to a coevolutionary race between the spurs of flowers and the proboscis of pollinators (Darwin, 1862; Wallace, 1867). Selection for longer spurs is expected if the spur is too short to match the length of the proboscis of the primary pollinator, while selection for a longer proboscis in the pollinator is expected as long spurs evolve because individuals with a long proboscis will be able to obtain more nectar than competitors with a short proboscis. However, the relative importance of coevolutionary interactions and pollinator shifts for the evolution of spur length remains unknown. Based on a comparative phylogenetic study, Whittall and Hodges (2007) suggested that interspecific differences in spur length in Aquilegia have evolved as a result of pollinator shifts rather than coevolution. Studies of intraspecific variation in corolla tube length and pollinator morphology have provided support for the hypothesis of coevolution in the South African herbs Zaluzianskya microsiphon (Anderson and Johnson, 2008) and Lapeirousia anceps (Pauw et al., 2009), while corresponding data rather suggested that pollinator shifts are largely responsible for spur length evolution in the deceptive orchid Disa draconis (Johnson and Steiner, 1997).
Spur length and other floral traits vary considerably among populations of the moth-pollinated orchid Platanthera bifolia, and particularly long-spurred woodland populations have been described as a separate subspecies (ssp. latiflora; Løjtnant, 1978; Sterner, 1986). Spur length manipulations have demonstrated that spur length influences both pollen removal and pollen receipt in P. bifolia (Nilsson, 1988; Boberg and Ågren, 2009), and a reciprocal transplant experiment suggested that differences in floral traits between a short-spurred grassland population and a long-spurred woodland population of P. bifolia had a genetic basis (Boberg and Ågren, 2009). Here, we investigated the adaptive significance of spur length in P. bifolia and whether among-population variation in spur length is consistent with coevolutionary interactions or pollinator shifts. We performed a survey of geographical variation in spur length and proboscis length of local pollinators across Scandinavia, and we conducted a reciprocal translocation experiment between a long-spurred and a short-spurred P. bifolia population in south-eastern Sweden to examine whether the relative pollination success of long-spurred and short-spurred plants differed between populations primarily serviced by long- and short-proboscis pollinators, respectively. More specifically, we asked (1) whether the spur length of P. bifolia varies among regions and habitats; (2) whether the proboscis lengths of the three most common flower visitors vary intraspecifically among regions; (3) whether among-population variation in spur length correlates with the proboscis length of local pollinators; (4) whether spur length correlates with other floral traits in an area with both long- and short-spurred populations; and (5) whether the pollination success of long-spurred plants is greater than that of short-spurred plants in the habitat where the primary pollinator has a long proboscis but not in the habitat where the main pollinator has a short proboscis, as would be expected if the spur length of local populations is sufficiently long to ensure mechanical fit and efficient pollination by local pollinators.
MATERIALS AND METHODS
Study system
Platanthera bifolia is a terrestrial orchid with a Eurasian distribution (Hultén and Fries, 1986). In northern Europe, it flowers in June and July. The plant produces two basal oval leaves at the base of a spike-like inflorescence. Each inflorescence contains 10–20 flowers, which open sequentially, basically to apically. All flowers open within a few days of each other and remain open for most of the flowering period. Nectar is secreted from unicellular hairs that cover the inside walls of the spur (Stpiczynska, 1997) and the flowers emit a strong scent at night (Tollsten and Bergström, 1993). The flower contains two pollinaria, one situated on each side of the spur entrance. Each pollinium contains hundreds of massulae (Nazarov and Gerlach, 1997). Platanthera bifolia is pollinated by hawkmoths (Sphingidae). Common hawkmoth flower visitors in Sweden are Deilephila porcellus, D. elpenor, Hyles gallii, Hyloicus pinastri and Sphinx ligustri (Nilsson, 1983, 1988). These species vary in proboscis length from approximately 18 to 45 mm, which should impose variation in selection on spur length of P. bifolia. When a moth inserts its proboscis into the spur to feed on nectar it contacts the viscidia that fold around the proboscis, and the pollinaria are removed when the moth withdraws from the flower. When a moth carrying pollinaria visits a flower, massulae attach to the sticky surface of the stigma, which is situated in three lobes around the spur entrance (Nilsson, 1983). Fruit set has been found to be pollen-limited in P. bifolia populations both on the Swedish mainland (Nilsson, 1983; Maad and Alexandersson, 2004) and on the island of Öland in south-eastern Sweden (E. Boberg, L. Xu and J. Ågren, unpubl. res.).
Spur length and pollinator morphology
To determine the correlation between geographical variation in spur length and in proboscis length of local pollinators, we measured spur length in 51 populations of P. bifolia (n = 3–200 plants per population, median n = 47) and the proboscis length of pollinating moths in 12 of the P. bifolia populations (n = 1–96 moths per population, median n = 6) across Sweden and Norway. For each plant sampled, spur length, defined as the distance from the spur mouth to the tip, was determined for two or three newly opened flowers positioned in the middle part of the inflorescence. Spur length was recorded to the nearest 0·1 mm using a digital calliper. We sampled eight different region/habitat categories. We sampled 12 populations in coniferous forest in northern Sweden, six populations in coniferous forest in central Sweden, four populations in coniferous forest on the island of Gotland in south-eastern Sweden and seven populations in deciduous woodland and 12 populations in semi-natural alvar grasslands on Öland in south-eastern Sweden. In northern Norway we sampled three populations in Calluna heathland, six populations in subalpine birch forest and one alpine population (see Supplementary Data Table S1 for population coordinates and sample sizes). Pollinating moths were observed and caught in populations of P. bifolia from June to July during 1973, 1980, 1984, 1985, 2002 and 2005. The moths that were caught either carried pollinaria or were observed pollinating flowers. We measured the proboscis length of hawkmoths from root to tip to the nearest 0·5 mm in the laboratory, and for each site we calculated the mean proboscis length of all hawkmoth specimens caught.
Intraspecific variation in proboscis length
To determine the magnitude of intraspecific variation in proboscis length of pollinators, we obtained specimens of the three important pollinator species S. ligustri, H. pinastri and D. porcellus collected with stationary light traps during June and July 2006 at nine sites in five geographical regions in southern and central Sweden. The proboscis length from root to tip was determined for 12–67 hawkmoths per region (1–42 hawkmoths per site) for the five regions Skåne, Blekinge, Öland, Gotland and Gästrikland/Uppland/Södermanland (see Supplementary Data Table S2 for sample sizes and the location of each collection site).
Local differentiation between grassland and woodland populations
To document the magnitude of morphological variation among populations on the island of Öland in south-eastern Sweden, where P. bifolia occurs in both grassland and woodland habitats and where distinct short-spurred and long-spurred populations have been identified (Sterner, 1986), we recorded plant height, flower length, flower width, spur length, stem height and the number of flowers of 531 individuals in seven grassland and five woodland populations (n = 12–74 per population). We determined plant height, defined as the distance from the base of the stem to the uppermost flower; flower width, defined as the horizontal distance between the outer tips of the left and right lateral sepals; flower length, defined as the vertical distance between the outer tips of the dorsal sepal and the labellum; spur length, defined as above; and stem height, defined as the distance between the base of the stem and the lowermost flower in the inflorescence.
Reciprocal translocation experiment
To establish the adaptive significance of differences in flower morphology and plant height in habitats that differ in pollinator composition, vegetation height and microclimate, we performed a translocation experiment at the sites of a short-spurred grassland population (Melösa) and a long-spurred woodland population (Ismantorp) on Öland in 2009. We included 184 plants from two short-spurred grassland populations (Melösa and Bårby) and two long-spurred woodland populations (Ismantorp and Gråborg) in the experiment. The short-proboscis pollinator D. porcellus is the primary pollinator of P. bifolia in grassland populations on Öland, while the long-proboscis hawkmoth S. ligustri is the primary pollinator in woodland populations. Grassland populations grow on thin, sandy soils with relatively low vegetation, and are exposed to the sun and often strong winds, while woodland populations grow on deep, clay-rich soils with tall grass and herbaceous vegetation in an environment that is shadier and less windy. This experiment primarily tested whether the relative pollination success of the two focal populations [short-spurred (Melösa) and long-spurred (Ismantorp)] differed between the two sites, such that the pollination success of the local population would be higher than that of the non-local population, at least at the site of the long-spurred population. We included two additional populations to determine whether differences in the relative pollination success of the two focal populations could be generalized to other long- and short-spurred populations. Plants were bagged in the bud stage until the date of the experiment. Woodland populations of P. bifolia start to flower about 10 days earlier than grassland populations (E. Boberg and J. Ågren, unpubl. res.). The translocation experiment was conducted during the period when flowering of woodland and grassland populations overlaps. To reduce variation in display size caused by differences in flowering time, we removed eight open flowers from each plant originating from woodland populations and eight flower buds from each plant originating from grassland populations. This resulted in a mean of nine open flowers per plant at the time of the experiment, with no statistically significant effect of population (two-way ANOVA, F3,164 = 0·28, P = 0·8), site (F1,164 = 1·3, P = 0·26) or their interaction (F3,164 = 2·21, P = 0·09). Plants originating from woodland populations were taller, with larger flowers (longer labellum, lateral sepal and dorsal sepal) and had longer spurs than had plants from grassland populations (significant effect of population in two-way ANOVA, P < 0·05, no statistically significant site × population interaction Supplementary Data Tables S3 and S4). In addition, the Melösa population was taller and produced longer spurs than did the other short-spurred population (Bårby; Tukey HSD P < 0·05); labellum length, lateral sepal length and dorsal sepal length did not differ significantly between the two short-spurred populations. Plants included in the translocation experiment were cut so that the inflorescence with stem and the two basal leaves were intact and the cut stems were inserted in Falcon tubes filled with floristic foam. At each site, 92 plants were randomly distributed at nodes within a quadratic grid containing 400 nodes in total, each separated by 0·5 m. Plants were left in the field for three subsequent nights, and did not show any signs of wilting during the course of the experiment. Pollen receipt, quantified as the number of massulae deposited in flowers, was recorded after each night of exposure to flower visitors. Massula diffuses into the stigmatic surface and become invisible after approximately 24 h (E. Boberg and J. Ågren, unpubl. res.). Total pollen receipt of experimental plants was therefore quantified as the sum of deposited massulae recorded after the first, second and third nights. Pollen removal was quantified at the end of the experiment as the total number of pollinia removed from each plant.
Data analyses
We used mixed-model ANOVA to assess the effects of region/habitat (fixed factor) and population nested within region/habitat (random factor) on spur length. We calculated the correlation between pollinator proboscis length and plant spur length based on population means. To determine whether proboscis length can explain variation in spur length also when controlling for large-scale climatic factors, we included latitude as a proxy for climate as an additional predictor in a multiple regression model. For the hawkmoth specimens collected with stationary light traps, we used mixed-model ANOVA to assess the effects of region (fixed factor) and collection site nested within region (random factor) on proboscis length separately for each species. To examine the main axes of variation in morphology in the populations of P. bifolia on Öland, we performed principal components analysis based on the correlation matrix for the six traits scored (plant height, stem height, number of flowers, flower width, flower length and spur length).
Because pollen receipt and pollen removal were low and highly variable in the translocation experiment, we treated pollen receipt and pollen removal as binomial variables (0, 1) at the level of the individual plant in the analysis of pollination success. The effects of population of origin, translocation site and their interaction on pollen receipt and pollen removal were analysed with generalized linear models with a binomial error distribution. To test for local adaptation, this analysis was conducted including only the two focal populations originating from the sites of the experiment. To test for general differences of adaptive significance between short-spurred and long-spurred populations, an analysis including all four populations was conducted. In the former analysis, contrasts were examined to determine whether the local population outperformed the non-local population at each of the two sites, and in the latter analysis contrasts were examined to determine whether long-spurred populations outperformed short-spurred populations at the site of the long-spurred population, and whether the reverse was true at the other site.
Mixed model ANOVA (PROC MIXED), two-way ANOVA (PROC GLM) and generalized linear models (PROC GENMOD) were analysed in SAS 9·3 (SAS Institute). The statistical significance of the random factor in mixed-model ANOVA was estimated with the likelihood ratio test (Littell et al., 1996). The difference in –2 residual log likelihood between models with and without the random factor was compared using the χ2 test with a table value corresponding to a one-tailed test with one degree of freedom (Sokal and Rohlf, 1995).
RESULTS
Spur length and pollinator morphology
Pollinators of P. bifolia varied among regions/habitats. Platanthera bifolia populations in grassland habitats on Öland, south-eastern Sweden, were primarily pollinated by the short-proboscis hawkmoth D. porcellus (22 of 30 pollinator observations; mean ± SD proboscis length 17·9 ± 1·0 mm, n = 22, pooled over populations), whereas populations in deciduous woodland habitats on Öland were primarily pollinated by the long-proboscis pollinator S. ligustri (52 of 68 pollinator observations; proboscis length 39·1 ± 2·2 mm, n = 52). The relatively few observations from populations in coniferous forest habitats suggest that the medium-proboscis H. pinastri is a major pollinator (seven observations of H. pinastri, two observations of D. porcellus and four observations of S. ligustri; proboscis length of H. pinastri 30·2 ± 2·5 mm, n = 7), while populations in subalpine habitats were pollinated by the small geometrid moth Entephria caesiata (103 of 103 pollinator observations; proboscis length 6·76 ± 0·61 mm, n = 103; Fig. 1). Of the total of 215 moths that were caught, 133 carried pollinaria of P. bifolia.
Fig. 1.
Mean spur length and proboscis length of pollinating moths in Platanthera bifolia populations in different regions/habitats (± SD). Deilephila porcellus was the primary pollinator of grassland populations of P. bifolia, Sphinx ligustri was the primary pollinator of populations in deciduous forest, Hyloicus pinastri was the primary pollinator of populations in coniferous forest and Entephria caesiata was the primary pollinator of populations in subalpine birch forest. Populations in (A) subalpine birch forest in northern Norway, (B) coniferous forest in northern Sweden, (C) coniferous forest on the island of Gotland, (D) deciduous forest on the island of Öland and (E) grasslands on the island of Öland.
Spur length varied among regions/habitats (F7,43·6 = 84·4, P < 0·0001; Fig. 2) and was positively related to the mean proboscis length of local pollinators (Fig. 3). Woodland populations on Öland had the longest spurs, whereas the shortest spurs were observed in grassland populations on Öland and in northernmost Norway (Fig. 2). Mean spur length and mean proboscis length were strongly and positively correlated (r = 0·93, n = 12, P < 0·001). Proboscis length was a significant predictor of spur length also in a multiple regression model that in addition included latitude as an independent variable (standardized partial regression coefficient: proboscis length, b = 8·8, t = 8·1, P < 0·001; latitude, b = 2·2, t = 2·0, P = 0·07).
Fig. 2.
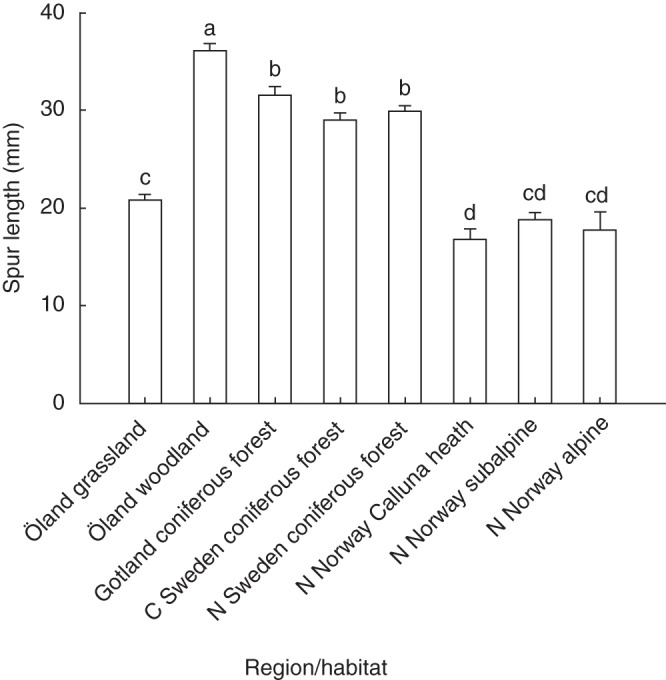
Spur length of Platanthera bifolia in different regions/habitats (± SE; least squares means extracted from mixed-model ANOVA). Different letters indicate statistically significant differences (Tukey–Kramer HSD test, P < 0·05).
Fig. 3.
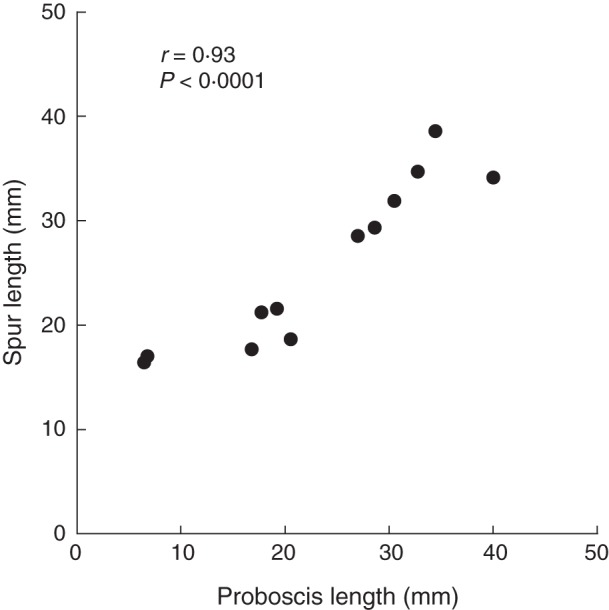
Relationship between spur length of Platanthera bifolia populations (n = 21–129 plants per population) and proboscis length of pollinating moths (n = 1–96 moths per population). Each symbol represents the population mean trait value.
Intraspecific variation in proboscis length
Intraspecific variation in proboscis length of three common pollinators of P. bifolia was limited, and variation among regions and collection sites reached marginal statistical significance only for one of the species (S. ligustri, region, F2,2·12 = 1·44, P = 0·40; collection site, χ2 = 0·3, d.f. = 1, P = 0·58; H. pinastri, F4,6·47 = 4·23, P = 0·05, χ2 = 2·5, d.f. = 1, P = 0·11; D. porcellus, F4,4·26 = 3·72, P = 0·11, χ2 = 0·1, d.f. = 1, P = 0·75; Fig. 4). Across all collection sites, the mean proboscis length ranged from 35·7 to 38·9 mm in S. ligustri, from 28·7 to 32·3 mm in H. pinastri and from 17·5 to 19·3 mm in D. porcellus.
Fig. 4.
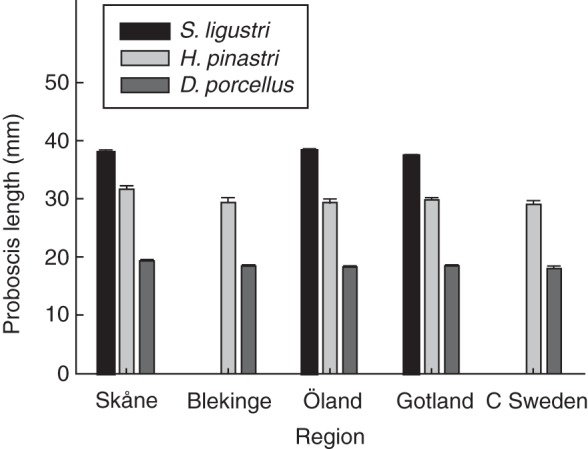
Proboscis length of Sphinx ligustri, Hyloicus pinastri and Deilephila porcellus collected in five different regions of southern and central Sweden (± SE; least squares means extracted from mixed-model ANOVAs). See Supplementary Data Table S2 for locations of collection sites.
Local differentiation between grassland and woodland populations
The populations investigated on the island of Öland consisted of two groups that were clearly separated morphologically (Fig. 5). In the principal components analysis, the first axis (PC1) explained 70·3 % of the variation and mainly represented variation in plant height, spur length, stem height and flower length and width (loadings ranging from 0·85 to 0·93; loading number of flowers 0·43). The second axis (PC2) explained 15·3 % of the variation and mainly represented number of flowers (loading 0·87; all other traits had absolute loadings <0·3). Long-spurred woodland populations and short-spurred grassland populations were clearly separated along PC1, but not along PC2 (Fig. 5). In woodland populations the plants were tall and produced large flowers with long spurs, while in grassland populations the plants were short and produced small flowers with short spurs; no consistent differences in number of flowers were recorded between long- and short-spurred populations (Supplementary Data Table S5). In addition, spur length and other floral traits also varied among populations of each of the two spur-length categories (range of population mean spur length: short-spurred populations, 19·2–23·2 mm; long-spurred populations 34·5–41·2 mm; Supplementary Data Table S5).
Fig. 5.
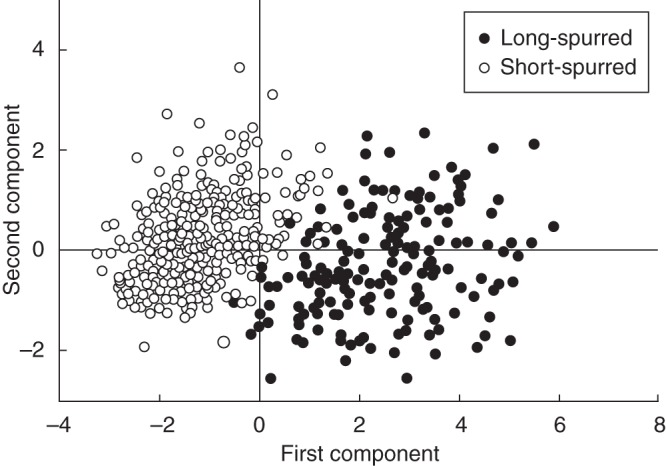
Score plot of a principal components analysis of variation in six traits (plant height, stem height, number of flowers, flower width, flower length and spur length) among 531 individuals in seven short-spurred grassland and five long-spurred woodland populations of Platanthera bifolia on the island of Öland, grouped according to population category (open symbols denote plants from short-spurred populations; filled symbols denote plants from long-spurred populations). The first two axes represent 86 % of the total variation in the examined traits.
Reciprocal translocation experiment
As predicted, the pollination success of long-spurred populations tended to be higher than that of short-spurred populations at the site of the long-spurred Ismantorp population, while no difference in pollination success was recorded at the site of the short-spurred Melösa population. In the analysis of the two focal populations, the long-spurred Ismantorp population was more likely to be pollinated at Ismantorp than was the short-spurred Melösa population (contrast χ2 = 11·4, d.f. = 1, P = 0·0007), while equal proportions of plants of the two populations were pollinated at Melösa (Fig. 6; significant site × population interaction, χ2 = 6·0, d.f. = 1, P = 0·014). The proportion of plants from which pollen was removed tended to be higher among the long-spurred Ismantorp plants than among the short-spurred Melösa plants at both sites, but this difference only approached statistical significance (χ2 = 3·4, d.f. = 1, P = 0·06), and no effects of site (χ2 = 0·3, d.f. = 1, P = 0·61) or site × population interaction (χ2 = 0·1, d.f. = 1, P = 0·74; Fig. 6) were detected.
Fig. 6.

(A) Proportion of plants with pollinaria removed and (B) proportion of plants receiving pollen after being exposed to flower visitors for three subsequent nights at the site of the short-spurred Melösa (Me) population and the site of the long-spurred Ismantorp (Is) population. Plants from the short-spurred grassland populations at Melösa (open symbols, solid lines) and Bårby (open symbols, dashed lines) and from the long-spurred woodland populations at Ismantorp (filled symbols, solid lines) and Gråborg (filled symbols, dashed lines) were translocated to each of the two sites.
The analysis that also included two additional populations suggested that long-spurred populations in general had an advantage at the woodland Ismantorp site, but the results were somewhat more variable. Pollen receipt was affected by population of origin (χ2 = 9·6, d.f. = 3, P = 0·02) but not by site (χ2 = 3·6, d.f. = 1, P = 0·06), and the site × population interaction only approached statistical significance (χ2 = 6·4, d.f. = 3, P = 0·09; Fig. 6). Contrasts examined separately by site indicated that long-spurred populations were more likely to be pollinated than short-spurred populations at Ismantorp (contrast Gråborg and Ismantorp with Bårby and Melösa, χ2 = 7·9, d.f. = 1, P = 0·005), but not at Melösa (χ2 = 1·1, d.f. = 1, P = 0·29). Pollen removal was not significantly affected by population of origin (χ2 = 4·1, d.f. = 3, P = 0·26), site (χ2 = 0·5, d.f. = 1, P = 0·47) or the population × site interaction (χ2 = 0·2, d.f. = 3, P = 0·98; Fig. 6).
DISCUSSION
In this study, we have documented geographical variation in spur length and predominant pollinator of the moth-pollinated orchid P. bifolia, and a correlation between spur length and proboscis length of local pollinators. We have further demonstrated that, at its home site, the pollination success of a long-spurred population was higher than that of a short-spurred population, while no significant difference was recorded at the home site of the short-spurred population. The results are consistent with the hypothesis that intraspecific variation in spur length reflects adaptive evolution in response to pollinator-mediated selection for longer spurs when spur length is insufficient to match the proboscis length of local pollinators.
We have demonstrated that the species identity of the primary pollinators varies across the distributional range of P. bifolia and that the mean spur length matches the mean proboscis length of local moth pollinators. The results are consistent with the hypothesis that pollinator shifts have driven large-scale population differentiation in spur length in this nectar-producing plant. An alternative hypothesis of pollinator-mediated floral evolution is diversification through the process of coevolution (Anderson and Johnson, 2008; Pauw et al., 2009). We found that intraspecific variation in proboscis length of three important pollinators of P. bifolia was very limited among and within regions and did not match the large variation in spur length observed in P. bifolia across Scandinavia or within the limited area of Öland. This suggests that spur length of P. bifolia has mainly evolved in response to variation in the composition of the local pollinator assemblage and that coevolution is likely to explain much less of the variation observed.
Correlations between spur length and other measures of flower size and inflorescence height documented in the present and previous studies (Boberg and Ågren, 2009) suggest that some of the among-population variation in spur length may reflect variation in abiotic conditions, overall size or pollinator-mediated selection on correlated traits. However, there was no clear latitudinal trend in spur length variation. The southernmost region examined (Öland) harboured both the most long-spurred and very short-spurred populations, and proboscis length of local pollinators remained a significant predictor of spur length when controlling for variation in latitude. Moreover, both the results of reciprocal transplantation of whole plants between a woodland and grassland population on Öland (Boberg and Ågren, 2009) and observations of among-year variation in spur length (Maad, 2000) suggest that environmental effects on spur length are limited compared with the differences documented between regions and between long- and short-spurred populations on Öland. Finally, the results of experimental manipulations suggest that spur length (Nilsson, 1988), but not perianth size (Boberg and Ågren 2009), is the direct target of pollinator-mediated selection in P. bifolia. Thus, although effects of abiotic factors and selection on correlated traits may contribute to variation in spur length, they are unlikely to be the sole causes of the differences in spur length observed among populations.
Correlations between among-population variation in spur length or corolla tube length and differences in pollinator species composition have been reported also in other insect-pollinated species. Robertson and Wyatt (1990) found that the difference in spur length between two butterfly-pollinated populations of Platanthera ciliaris corresponded to a difference in the primary pollinator species and its proboscis length, while Johnson and Steiner (1997) showed that the mean spur length of the deceptive orchid D. draconis matched the proboscis length of the local fly pollinator species. Measurements of museum specimens of plants and hawkmoths revealed that regional variation in corolla tube length in Gladiolus longicollis was correlated with the proportion of long-tongued hawkmoths in the collections from different regions (Anderson et al., 2010a). Taken together, these studies indicate the potentially strong influence of geographical variation in pollinator assemblage on the evolution of floral traits.
As predicted, the translocation experiment conducted on Öland showed that, at its home site, the pollination success of the long-spurred Ismantorp population was higher than that of the short-spurred Melösa population, while at Melösa no significant difference was recorded. This is consistent with the assumption that the spurs of the Melösa population are sufficiently long for efficient pollination in the home environment, but too short for efficient pollination at the site of the long-spurred population. The pollination success of the two additional populations included in the experiment was largely consistent with a general advantage of long-spurred populations at the site of the long-spurred Ismantorp population and with smaller differences at the site of the short-spurred Melösa population.
Woodland plants are taller and produce flowers with a larger perianth and with longer spurs [Supplementary Data Table S5], and several traits may thus have contributed to their higher pollination success at the site of the long-spurred Ismantorp population. The proboscis length of the principal pollinator of woodland populations is nearly 20 mm longer than that of the principal pollinator of grassland populations of P. bifolia on Öland, and the optimal spur length can therefore also be expected to be longer. Evidence of pollinator-mediated selection on spur length in P. bifolia has been detected in previous studies. Experimental shortening of the spur in long-spurred woodland populations on Öland reduced pollen removal and receipt (Nilsson, 1988; Boberg and Ågren, 2009), and a positive relationship between spur length and pollen removal and fruit set has been documented in natural populations of P. bifolia both on the Swedish mainland (Maad, 2000; Maad and Alexandersson, 2004) and on Öland (E. Boberg, L. Xu and J. Ågren, unpubl. res.). A field experiment showed that perianth size did not affect reproductive success in a woodland population of P. bifolia on Öland (Boberg and Ågren, 2009), suggesting that the difference in perianth size is less likely to have contributed to the difference in pollination success between the long-spurred woodland and the short-spurred grassland populations in the translocation experiment. The adaptive significance of plant height remains to be tested experimentally. Pollinator-mediated selection on inflorescence height can be expected to be stronger in tall than in short vegetation (Ågren et al., 2006; Sletvold et al., 2013), but experimental manipulations of both spur length and plant height would be required to assess their relative importance for variation in pollination success in the habitats examined here.
The translocation experiment provides a partial test of the hypothesis that the two reciprocally translocated populations are locally adapted. As predicted, the long-spurred population had an advantage at its home site in terms of higher pollination success compared with the short-spurred population. The results suggest that, at the site of the long-spurred population, benefits in terms of increased pollination success may balance costs associated with producing long spurs and a tall inflorescence. Because no such benefit was recorded at the site of the short-spurred population, the optimal spur length is likely to be shorter at this site. Opposing selection on flower size and inflorescence height is likely to be expressed through fitness components other than pollination success (cf. Zhang et al., 2013), and a full evaluation of the hypothesis that the morphological differences documented are adaptive would thus require that effects on overall fitness are considered.
This study has demonstrated wide variation in spur length among populations of P. bifolia and a correlation between plant and pollinator morphology. The results support the hypothesis that intraspecific variation in spur length reflects adaptive evolution in response to geographically varying pollinator-mediated selection, but do not by themselves say anything about when and where differences in spur length evolved. It would be interesting to examine the genetic relationships between long-spurred and short-spurred populations of P. bifolia. This would make it possible to determine whether particularly long- and short-spurred populations have evolved repeatedly, to estimate the timing of divergence in relation to recent glaciations in Northern Europe and to estimate current levels of gene flow between morphologically and phenologically differentiated populations. The wide variation in floral morphology observed in P. bifolia makes it an excellent study system for examining processes influencing floral evolution and reproductive isolation.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Per-Erik Betsholtz, Clas Källander, Göran Palmqvist and Nils Ryrholm for sending us hawkmoth specimens that allowed us to assess intraspecific variation in proboscis length among regions; Sonia and Lars-Ewe Larsson for help with fencing at the Melösa field site; Christina and Björn Svensson, The Royal Swedish Academy of Letters History and Antiquities, Börje Karlsson and the Swedish National Heritage Board for permission to work at the field sites; and Jonas Boberg for assistance in the field. Jeff Karron, Steve Johnson and two anonymous reviewers provided helpful comments on the manuscript. Permission to collect inflorescences of Platanthera bifolia was given by the County Administrative Board in Kalmar. This study was supported financially by grants from the Swedish Research Council to J. Ågren and L.A. Nilsson.
LITERATURE CITED
- Ågren J, Schemske DW. Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytologist. 2012;194:1112–1122. doi: 10.1111/j.1469-8137.2012.04112.x. [DOI] [PubMed] [Google Scholar]
- Ågren J, Fortunel C, Ehrlén J. Selection on floral display in insect-pollinated Primula farinosa: effects of vegetation height and litter accumulation. Oecologia. 2006;150:225–232. doi: 10.1007/s00442-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Johnson SD. The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution. 2008;62:220–225. doi: 10.1111/j.1558-5646.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Alexandersson R, Johnson SD. Evolution and coexistence of pollination ecotypes in an African Gladiolus (Iridaceae) Evolution. 2010a;64:960–972. doi: 10.1111/j.1558-5646.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Terblanche JS, Ellis AG. Predictable patterns of trait mismatches between interacting plants and insects. BMC Evolutionary Biology. 2010b;10:204. doi: 10.1186/1471-2148-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster WS. Evolution of plant pollination systems – hypotheses and tests with the neotropical vine Dalechampia. Evolution. 1993;47:1480–1505. doi: 10.1111/j.1558-5646.1993.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Baldwin BG. Switch from specialized to generalized pollination. Nature. 1998;394:632–632. [Google Scholar]
- Armbruster WS, Lee J, Baldwin BG. Macroevolutionary patterns of defense and pollination in Dalechampia vines: adaptation, exaptation, and evolutionary novelty. Proceedings of the National Academy of Sciences of the USA. 2010;106:18085–18090. doi: 10.1073/pnas.0907051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg E, Ågren J. Despite their apparent integration, spur length but not perianth size affects reproductive success in the moth-pollinated orchid Platanthera bifolia. Functional Ecology. 2009;23:1022–1028. [Google Scholar]
- Boyd A. Morphological analysis of Sky Island populations of Macromeria viridiflora (Boraginaceae) Systematic Botany. 2002;27:116–126. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Brink DE. Reproduction and variation in Aconitum columbianum (Ranunculaceae) with emphasis on California populations. American Journal of Botany. 1980;67:263–273. [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. Pollen transfer by hummingbirds and bumblebees, and the divergence of pollination modes in Penstemon. Evolution. 2003;57:2742–2752. doi: 10.1111/j.0014-3820.2003.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Clausen J, Keck DD, Hiesey WM. Experimental studies on the nature of species. I. Effect of varied environment on Western North American plants. Publication No. 520. Washington, DC: Carnegie Institution of Washington; 1940. [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing. London: Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Dodd ME, Silvertown J, Chase MW. Phylogenetic analysis of trait evolution and species diversity variation among angiosperm families. Evolution. 1999;53:732–744. doi: 10.1111/j.1558-5646.1999.tb05367.x. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 2004;35:375–403. [Google Scholar]
- Fenster CB, Martén-Rodriguez S, Schemske DW. Pollination syndromes and the evolution of floral diversity in Iochroma (Solanaceae) Evolution. 2009;63:2758–2762. doi: 10.1111/j.1558-5646.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Perfectti F, Bosch J, Camacho JPM. A geographic selection mosaic in a generalized plant-pollinator-herbivore system. Ecological Monographs. 2009;79:245–263. [Google Scholar]
- Harder LD, Johnson SD. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist. 2009;183:530–545. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Hereford J. A quantitative survey of local adaptation and fitness trade-offs. American Naturalist. 2009;173:579–588. doi: 10.1086/597611. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Castellanos MC, Medrano M. Geographical context of floral evolution. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Hultén E, Fries M. Atlas of North European vascular plants: north of the Tropic of Cancer. Königstein: Koeltz. 1986 [Google Scholar]
- Janzen DH. When is it coevolution? Evolution. 1980;34:611–612. doi: 10.1111/j.1558-5646.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Linder HP, Steiner KE. Phylogeny and radiation of pollination systems in Disa (Orchidaceae) American Journal of Botany. 1998;85:402–411. [PubMed] [Google Scholar]
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecology Letters. 2004;7:1225–1241. [Google Scholar]
- Kay KM, Sargent RD. The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annual Review of Ecology, Evolution, and Systematics. 2009;40:637–656. [Google Scholar]
- Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS One. 2008;3:e4010. doi: 10.1371/journal.pone.0004010. doi:10.1371/journal.pone.0004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for mixed models. SAS Institute Inc: Cary, NC; 1996. [Google Scholar]
- Løjtnant B. Nomenclatural notes upon Scandinavian orchids. Feddes Repertorium. 1978;89:13–18. [Google Scholar]
- Maad J. Phenotypic selection in hawkmoth-pollinated Platanthera bifolia: targets and fitness surfaces. Evolution. 2000;54:112–123. doi: 10.1111/j.0014-3820.2000.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Maad J, Alexandersson R. Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. Journal of Evolutionary Biology. 2004;17:642–650. doi: 10.1111/j.1420-9101.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Thomson JD. Going to great lengths: selection for long corolla tubes in an extremely specialized bat-flower mutualism. Proceedings of the Royal Society of London B, Biological Sciences. 2009;276:2147–2152. doi: 10.1098/rspb.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov VV, Gerlach G. The potential seed productivity of orchid flowers and peculiarities of their pollination systems. Lindleyana. 1997;12:188–204. [Google Scholar]
- Newman E, Anderson B, Johnson SD. Flower colour adaptation in a mimetic orchid. Proceedings of the Royal Society of London B, Biological Sciences. 2012;279:2309–2313. doi: 10.1098/rspb.2011.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Niet T, Johnson SD. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends in Ecology and Evolution. 2012;27:353–361. doi: 10.1016/j.tree.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Nilsson LA. Processes of isolation and introgressive interplay between Platanthera bifolia (L) Rich and P. chlorantha (Custer) Reichb. (Orchidaceae) Botanical Journal of the Linnean Society. 1983;87:325–350. [Google Scholar]
- Nilsson LA. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Nuismer SL, Thompson JN, Gomulkiewicz R. Coevolutionary clines across selection mosaics. Evolution. 2000;54:1102–1115. doi: 10.1111/j.0014-3820.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Nuismer SL, Gomulkiewicz R, Ridenhour J. When is correlation coevolution? American Naturalist. 2010;175:525–537. doi: 10.1086/651591. [DOI] [PubMed] [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. Flies and flowers in Darwin's race. Evolution. 2009;63:268–279. doi: 10.1111/j.1558-5646.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. A pollinator shift explains floral divergence in an orchid species complex in South Africa. Annals of Botany. 2014;113 doi: 10.1093/aob/mct216. 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher M. Evolutionary transitions in flower color. International Journal of Plant Sciences. 2008;169:7–21. [Google Scholar]
- Robertson JL, Wyatt R. Evidence for pollination ecotypes in the yellow fringed orchid, Platanthera ciliaris. Evolution. 1990;44:121–133. doi: 10.1111/j.1558-5646.1990.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Ågren J. Nonadditive effects of floral display and spur length on reproductive success in a deceptive orchid. Ecology. 2011;92:2167–2174. doi: 10.1890/11-0791.1. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Grindeland JM, Ågren J. Vegetation context influences the strength and targets of pollinator-mediated selection in a deceptive orchid. Ecology. 2013;94:1236–1242. doi: 10.1890/12-1840.1. [DOI] [PubMed] [Google Scholar]
- Smith SD, Ane C, Baum DA. The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae) Evolution. 2008;62:793–806. doi: 10.1111/j.1558-5646.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- Sun M, Gross K, Schiestl FP. Floral adaptation to local pollinator guilds in a terrestrial orchid. Annals of Botany. 2014;113 doi: 10.1093/aob/mct219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R, Rohlf R. Biometry. New York, NY: WH Freeman; 1995. [Google Scholar]
- Sterner R. Ölands kärlväxtflora. Lund, Sweden: Forskningsrådens förlagstjänst; 1986. [Google Scholar]
- Stpiczynska M. The structure of nectary of Platanthera bifolia L. Orchidaceae. Acta Societatis Botanicorum Poloniae. 1997;66:5–11. [Google Scholar]
- Strauss SY, Irwin RE. Ecological and evolutionary consequences of multispecies plant-animal interactions. Annual Review of Ecology, Evolution, and Systematics. 2004;35:435–466. [Google Scholar]
- Tollsten L, Bergström LG. Fragrance chemotypes of Platanthera (Orchidaceae) – the result of adaptation to pollinating moths? Nordic Journal of Botany. 1993;13:607–613. [Google Scholar]
- Vamosi JC, Vamosi SM. Key innovations within a geographical context in flowering plants: towards resolving Darwin's abominable mystery. Ecology Letters. 2010;13:1270–1279. doi: 10.1111/j.1461-0248.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- Wallace AR. Creation by law. Quarterly Journal of ScienceS140. 1867:471–486. [Google Scholar]
- Wasserthal LT. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Botanica Acta. 1997;110:343–359. [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–712. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Wilson P, Castellanos MC, Hogue JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndromes among penstemons. Oikos. 2004;104:345–361. [Google Scholar]
- Zhang F, Hui C, Pauw A. Adaptive divergence in Darwin's race: how coevolution can generate trait diversity in a pollination system. Evolution. 2013;67:548–560. doi: 10.1111/j.1558-5646.2012.01796.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



