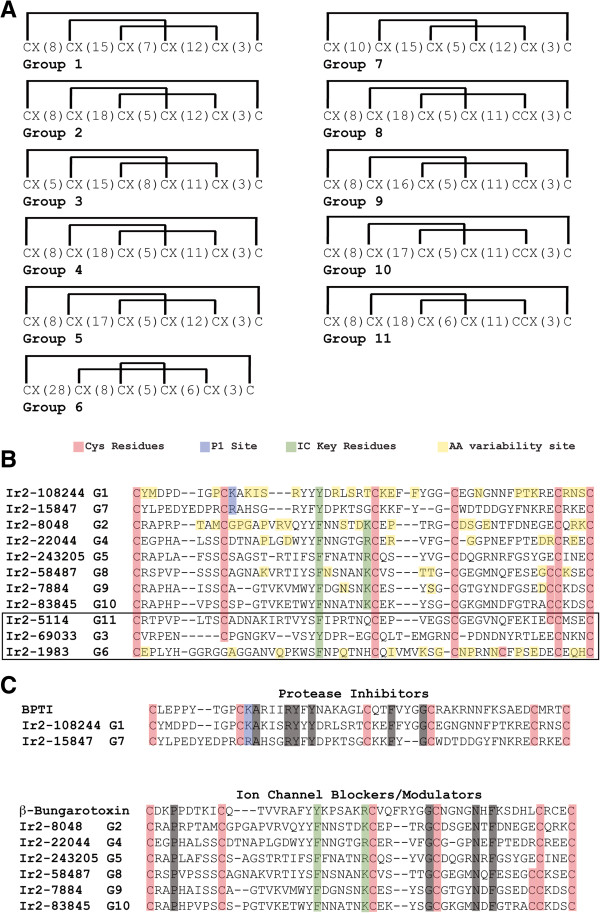
Figure 1.
I. ricinus Kunitz groups based on Cys motif. Kunitz peptides from the annotated transcriptome of I. ricinus were organized into 11 groups (G1-G11) based on their Cys motif (C = Cys and X = intra-Cys residues). The Cys motif for each group is depicted in Panel A along with their predicted disulfide bond patterns (in brackets). The alignment of one representative for each group (B) shows the conserved Cys residues (red), the positions of aa variability (yellow), and the key residues that interact with serine proteases (blue) and ion channels (green). The box around G3, G6 and G11 indicates that they do not contain all of the standard residues for serine protease (blue) or ion channel (green) interactions. Two separate alignments in Panel C depict the key conserved residues for serine protease inhibition (blue) using BPTI (UniProt: P00974) and ion channel blockage (green) using beta-bungarotoxin (UniProt: P00989). Residues highlighted in black are conserved.