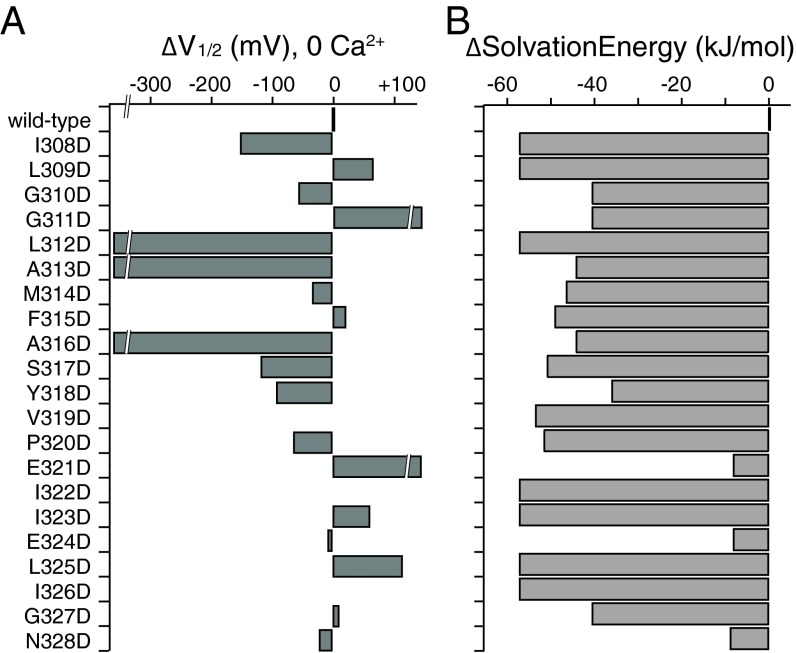
Fig. 7.
Similar changes in side-chain solvation energy resulted in significant differences in ΔV1/2 at different locations along BK S6. (A) Differences in V1/2 between the aspartate mutants and wild type, ΔV1/2, plotted for residues 308→328 along BK S6, in 0 Ca2+. V319D, I322D, and I326D did not produce measurable currents. (B) Differences in solvation energy between the aspartate side chain and the side chain of the endogenous residue at locations along S6 in BK.