Fig. 4.
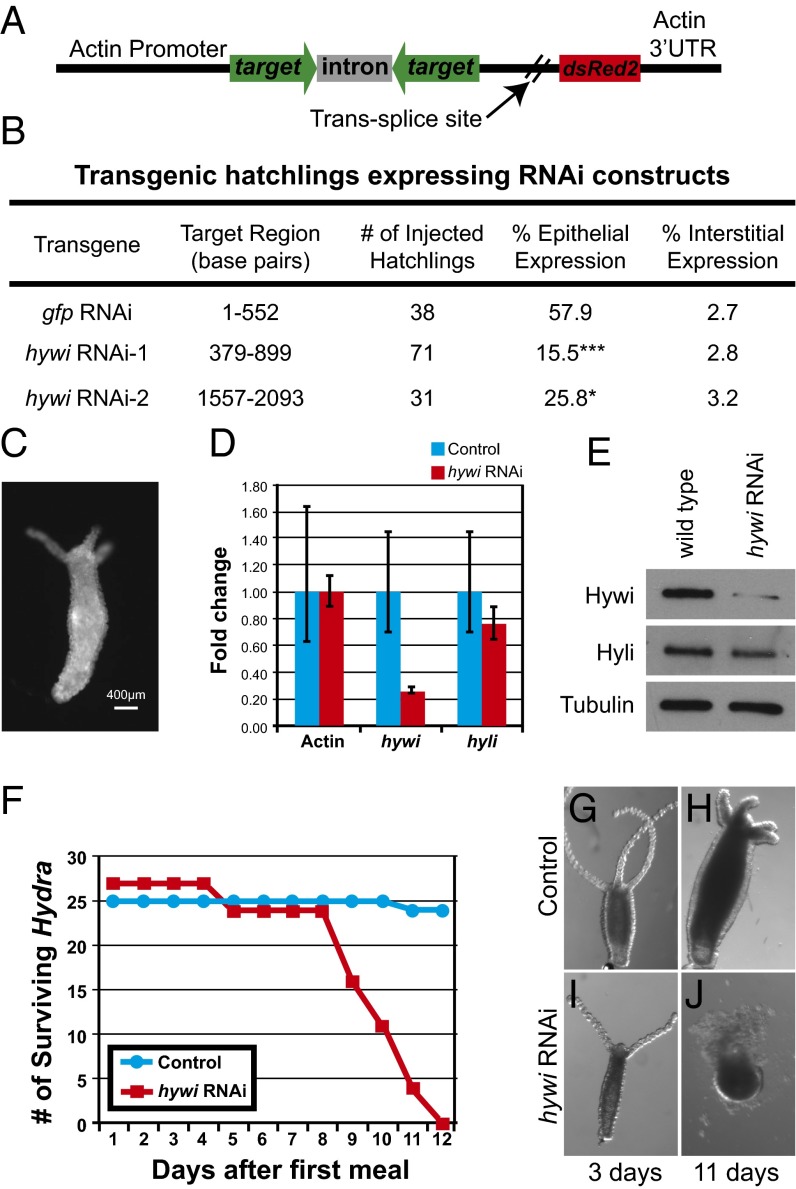
Hywi has an essential function in Hydra epithelial cells. (A) Schematic of the RNAi construct used to knock down hywi in the epithelial cells (ectoderm and endoderm). Expression of the transgene is driven by an actin promoter, which is active in all cells except the interstitial stem cells (SI Appendix, Fig. S7 A–C). The RNA hairpin (inverted repeats separated by an actin intron spacer) and the DsRed2 transcript are arranged in an operon configuration that is spliced apart after transcription. (B) Hydra embryos were injected with control (gfp RNAi) and hywi RNAi knockdown plasmids, and the percentage of injected hatchlings that have transgene expression was quantified. Significantly fewer hatchlings expressed the hywi knockdown transgene in the epithelium compared with the control; the P values for epithelial expression of the hywi RNAi constructs are 0.0001 (hywi RNAi-1) and 0.04 (hywi RNAi-2). (C) The hywi RNAi-1 transgene was stably incorporated into the interstitial lineage and underwent germline transmission (SI Appendix, Fig S8 D–F). This resulted in F1 Hydra expressing the transgene in both the ectodermal and endodermal epithelial layers, but not the interstitial stem cells (SI Appendix, Fig S8 G–M). (D) By qRT-PCR, the hywi mRNA levels (normalized to GAPDH) are reduced by ∼80% in transgenic F1 hatchlings (samples taken 7 d after hatchling), and (E) the protein levels are reduced. (D and E) By contrast, hyli RNA and protein levels are similar between control and hywi knockdown F1 animals. (F) hywi knockdown F1 animals all die between 8 and 12 d after eating their first meal, whereas control hatchlings are normal. (G and H) Control F1 animals (nontransgenic siblings) look normal after 11 d. (I and J) hywi knockdown F1 animals are initially normal, but lose epithelial integrity between 8 and 12 d after eating.