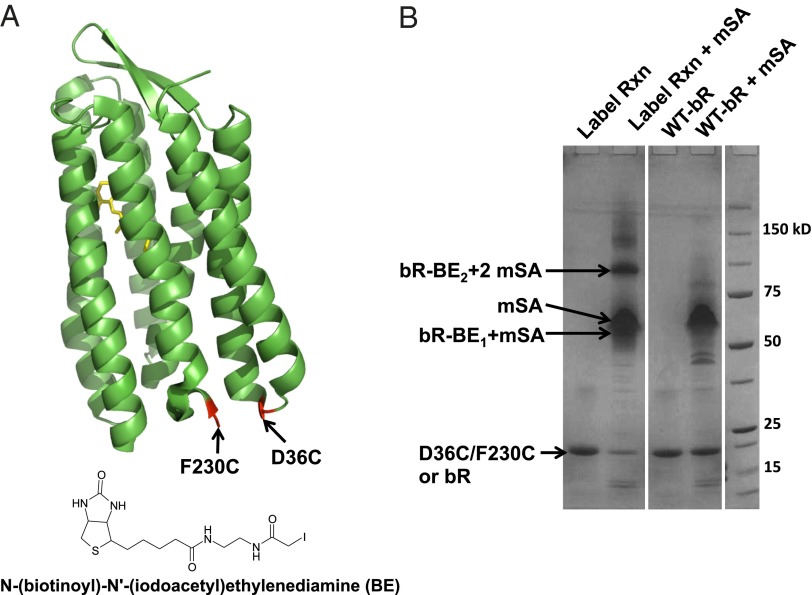
Fig. 2.
Biotinylation. (A) The positions of unique cysteines (shown in red) available for labeling on bR mutant D36C/F230C. The chemical structure of the labeling reagent, BE, is also shown. (B) SDS/PAGE-based gel shift assay to assess labeling efficiency. Lane 1 (Label Rxn): bR-D36C/F23C after the labeling reaction. Lane 2 (Label Rxn + mSA): The protein in lane 1 after the addition of mSA. Lane 3 (WT-bR): Wild-type bR. Lane 4 (WT-bR + mSA): Wild-type bR after the addition of mSA. The positions of the unbound protein (D36C/F230C or bR), mSA, the singly bound protein (bR-BE1 + mSA), and the doubly bound protein (bR-BE2 + mSA) are shown.