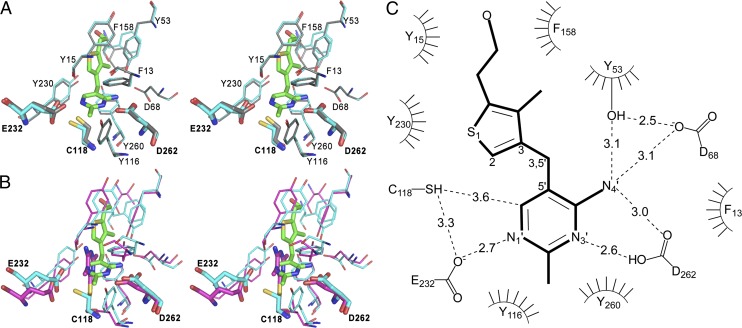
Fig. 3.
The N. gruberi thiaminase I active site. (A) Stereoview of the superposition of apo (gray; PDB ID code 4HCW) and holo (cyan; 3-dzThi, green; PDB code 4HCY) active sites of Ng-thiaminase. (B) Stereoview of the superposition of Ng-thiaminase (cyan; PDB ID code 4HCY) in complex with 3-dzThi (green) with the active site of Bt-thiaminase in complex with covalently bound inhibitor, Pyd [magenta; PDB ID code 4THI (31)]. For clarity, only Asp (Ng-262, Bt-272), Cys (Ng-118, Bt-113), and Glu (Ng-232, Bt-241) are labeled. (C) Schematic view of the Ng-thiaminase active site. Hydrogen bonds are represented by dashed lines, and residues involved in hydrophobic contacts to the ligand are represented by a curved comb.