Fig. 5.
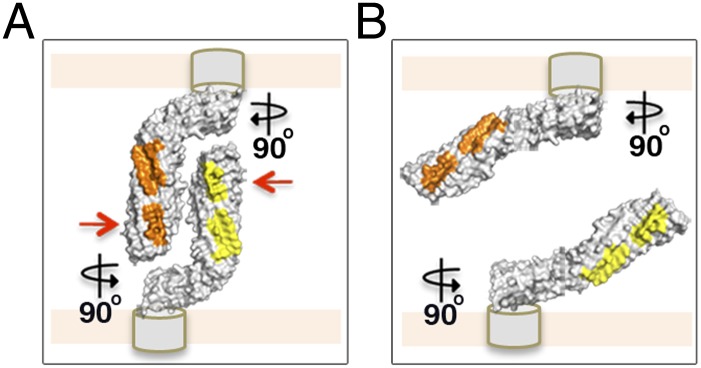
Model of a molecular “Velcro-like” mechanism of bacterial cell aggregation mediated by Ag43a self-assembly in a head-to-tail conformation. (A) Close-up view of the self-associating α43a molecules rotated 90°. The α43a functional domains are predicted to interact with the β-translocating domain (depicted as gray cylinders) using the foot of the L, which will position the long stem of the protein almost perpendicular to the cell surface, making the interacting surfaces (shown in yellow and orange) easily accessible to neighboring molecules. Mutation of the interacting residues in interface 1 (red arrows) completely abolishes Ag43-mediated cell aggregation. (B) Loss of the overall L shape in α43a likely changes the positioning of the long stem of the protein relative to the cell surface, from an easily accessible perpendicular arrangement to a nearly parallel layout, compromising the ability of α43a to interact with Ag43 molecules on adjacent bacterial cells.
