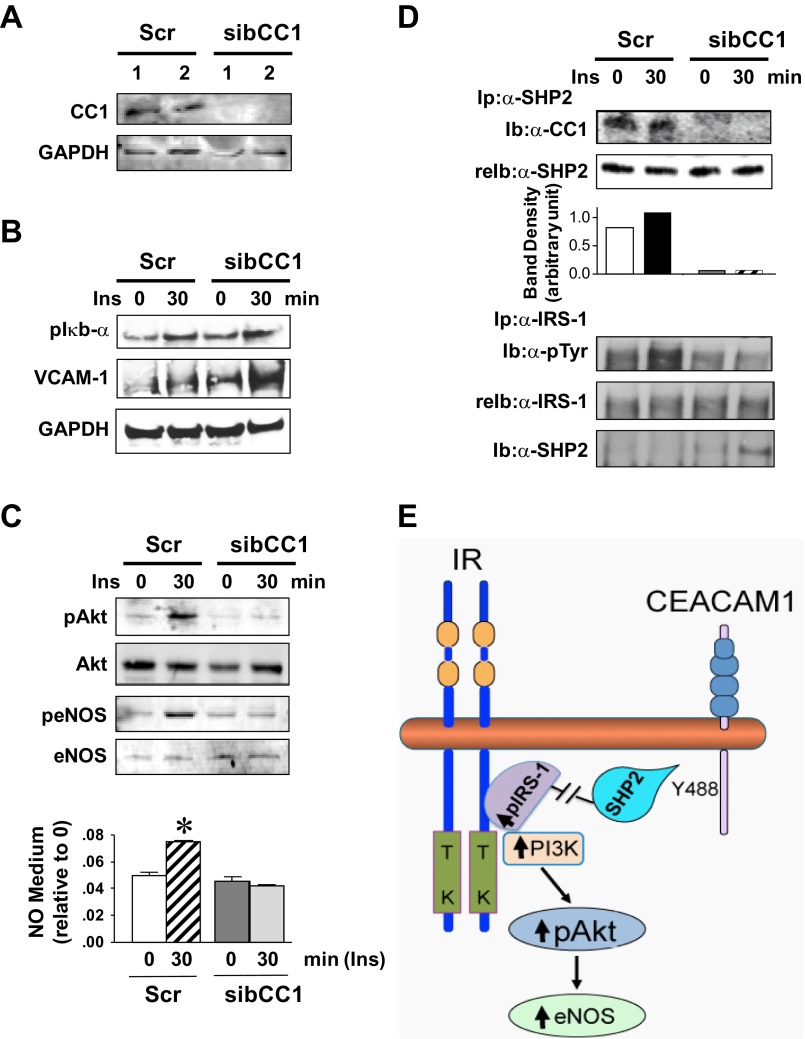
Fig. 5.
Cell autonomous effect of Ceacam1 deletion on Akt/eNOS activation in cultured bovine aortic endothelial (BAE) cells. A: BAE cells were transfected with scrambled (Scr) or bovine CEACAM1-specific siRNA (sibCC1) prior to WB analysis of lysates with anti-CEACAM1 antibody to assess loss of CEACAM1. B: cells were serum starved overnight and treated with insulin (100 nM) for 0–30 min and their lysates analyzed for p-Iκbα and VCAM-1 content. C: lysates were analyzed for Akt/eNOS phosphorylation and cell media assayed for NO level. Gels represent 3 different experiments, and NO data are represented as means ± SE of ≥3 experiments. *P < 0.05 vs. time 0 per group. D: some lysates were immunoprecipitated (Ip) with α-SHP2 (Src homology tyrosine phosphatase 2) prior to being immunoblotted (Ib) with α-CC1 (top) or with α-IRS-1 (bottom) prior to being immunoblotted sequentially with α-SHP2 to assess binding, and with α-phosphotyrosine (α-p-Tyr) antibody to assess phosphorylation. Gels represent 3 different experiments. E: working model summarizing the mechanisms underlying the cell autonomous effect of CEACAM1 on Akt/eNOS activation.