Abstract
Background:
Burkitt lymphoma (BL) is a rare, highly aggressive B-cell malignancy treated most successfully with brief-duration, high-intensity chemotherapeutic regimens. The benefit of the addition of rituximab to these regimens remains uncertain. We sought to examine the effectiveness of chemotherapy with and without rituximab in patients with BL.
Methods:
This study is a retrospective cohort study of all adult patients with BL diagnosed and treated with modern, dose-intense chemotherapeutic regimens from 1998–2008 at two tertiary care institutions. All cases were confirmed by application of WHO 2008 criteria by hematopathologists. Medical records were reviewed for patient-, disease-, and treatment- related factors as well as treatment response and survival. Factors associated with survival were analyzed using Cox proportional hazards modeling.
Results:
A total of 35 patients were analyzed: 18 patients received rituximab with chemotherapy (R-chemo) and 17 received chemotherapy (chemo) alone. The median age was 42 (range 20–74 years); 57% were male; 71% had Ann Arbor Stage IV disease; 33% had central nervous system involvement; 78% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. R-chemo was associated with significantly longer overall survival (OS) than chemo alone (5-year OS 70% and 29%, respectively, p = 0.040). On multivariate regression analysis, poor performance status and central nervous system involvement were associated with poorer survival.
Conclusions:
The addition of rituximab to chemotherapy was associated with improved OS in patients with Burkitt lymphoma. Poor performance status and central nervous system involvement were prognostically significant on multivariate analysis.
Keywords: Burkitt lymphoma, chemotherapy, rituximab, survival
Introduction
Burkitt lymphoma (BL) is a rare, highly aggressive, mature B-cell neoplasm, accounting for approximately 1–2% of all adult non-Hodgkin lymphoma (NHL) patients diagnosed in North America and Western Europe. The optimal treatment of BL remains undefined. Conventional NHL regimens such as CHOP (Cyclophosphamide, doxorubicin, vincristine, and prednisone) yield unsatisfactory results in BL, with complete response (CR) rates of 30–70% and long-term remission rates between 0 and 30% [Blayney, 2003; Kantarjian et al. 1990]. High-intensity, brief duration regimens yield CR rates of 70–90%, and long-term event-free survival (EFS) rates between 45% and 97% [Evens et al. 2013; Barnes et al. 2011; Dunleavy et al. 2011; Fayad et al. 2007; Thomas et al. 1999, 2006; Rizzieri et al. 2004; Mead et al. 2002]. In contrast to other B-cell lymphomas, the impact of rituximab in combination with chemotherapy in BL is unclear, with some studies finding no benefit and others strongly suggestive of benefit [Wästerlid et al. 2011, 2013; Todeschini et al. 2012; Barnes et al. 2011; Thomas et al. 2006]. One randomized trial, presented in abstract form, reported an overall survival (OS) benefit of the addition rituximab to intensive chemotherapy [Ribrag et al. 2012].
Most BL regimens have been evaluated in nonrandomized single-center studies, raising the question of generalizability of results to the general population. In addition, evolution in the classification of BL over the past 30 years has made cross-trial comparisons even more problematic, with clinically heterogeneous histologies such as B-cell acute lymphoblastic leukemia and B-cell lymphoma, unclassifiable, with features intermediate between BL and diffuse large B cell lymphoma (BCL-U) included [Bennett et al. 1985; Dave et al. 2006; Harris et al. 1999; Hummel et al. 2006; The Non-Hodgkin’s Lymphoma Classification Project, 1982; Swerdlow et al. 2008].
We therefore studied a cohort of patients with BL as defined by WHO 2008 criteria and diagnostically confirmed using rigorous contemporary pathologic techniques, and treated at two US tertiary care centers over a 10-year period (1998–2008), to assess the impact of different chemotherapy regimens and rituximab on outcomes.
Patients and methods
Patients
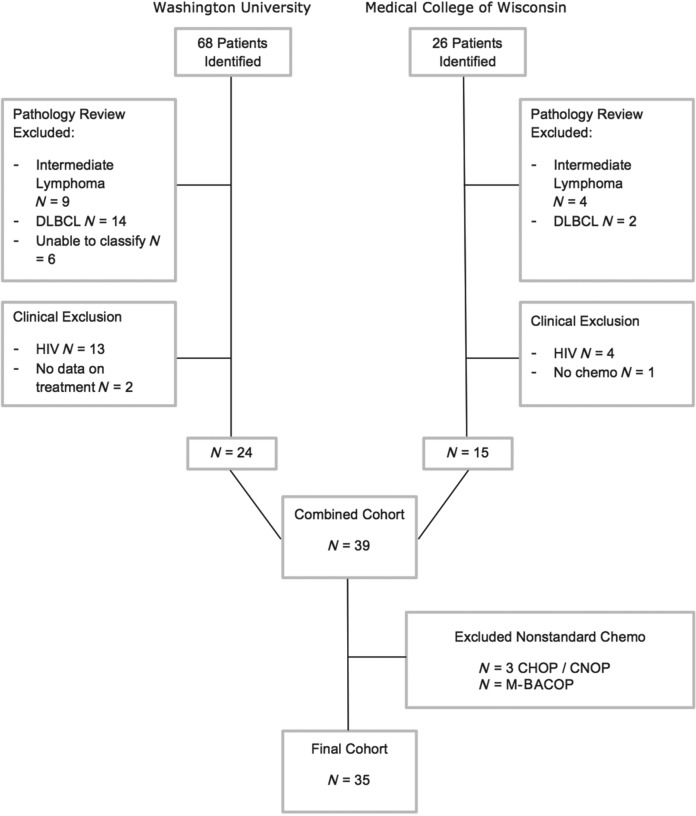
With the approval of the respective human studies committees, we identified all patients with BL who were diagnosed and treated at Washington University School of Medicine or the Medical College of Wisconsin from 1998–2008. To do this, we initially identified all patients with a clinical or pathologic diagnosis of sporadic small noncleaved lymphoma, BL or Burkitt-like lymphoma, then restricted our cohort to those who met clinical and pathologic criteria as outlined below (see Figure 1).
Figure 1.
Cohort assembly. DLBCL, diffuse large B-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; CNOP, cyclophosphamide, mitroxantone, vincristine, prednisone; M-BACOP, methotrexate, bleomycin, doxorubicin, cycylophosphamide, vincristine and dexamethasone.
Cases at Washington University School of Medicine were identified from Barnes-Jewish Hospital Oncology Data Service cancer registry and the pathology database. Patients from the Medical College of Wisconsin were identified using chemotherapy and pathology databases. Patients who received no chemotherapy or non-dose-intense chemotherapeutic regimens such as CHOP or for whom information on initial chemotherapy was not available were excluded from the analyses [Wästerlid et al. 2013]. Patients with HIV were excluded.
Pathologic confirmation of diagnosis
All cases were reviewed by subspecialty-trained hematopathologists, including available H&E-stained sections, cytology, immunohistochemistry, flow cytometry, cytogenetics, and fluorescence in situ hybridization (FISH). Utilizing the World Health Organization (WHO) 2008 classification, BL cases had to meet all of the following conditions: small or medium sized cells with monotonous morphology, proliferative index > 95% by MIB-1 or Ki-67 immunohistochemical staining, bcl-2 staining negative or weak, immunophenotype otherwise consistent with BL, and c-myc rearrangement documented by karyotype or FISH [Swerdlow et al. 2008]. Cases diagnosed as diffuse large B-cell lymphoma (DLBCL) or BCL-U (with features intermediate between BL and DLBCL, including the so-called ‘double-hit’ lymphomas which are positive for both c-myc and bcl-2 translocations) were excluded.
Clinical data
The medical record of each patient was reviewed to obtain demographic information, staging information, laboratory data, chemotherapy, response to therapy, relapse and survival. Lactate dehydrogenase (LDH) and uric acid values obtained prior to initiation of chemotherapy were included for analysis. Given differences in institutional reference ranges, LDH and uric acid were dichotomized as normal versus greater than the respective institution’s upper limit of normal (ULN) for analyses. Eastern Cooperative Oncology Group (ECOG) performance status (PS) at the time of diagnosis was determined. Central nervous system (CNS) involvement was defined as the presence of positive cerebrospinal fluid (CSF) cytology or flow cytometry or CSF cytology reported as ‘suspicious’ in concert with elevated protein levels and clinical findings consistent with CNS involvement. Bone marrow involvement was confirmed by bone marrow aspirate or trephine biopsy. Chemotherapeutic regimen was determined by the regimen received for the majority of cycles, allowing that some patients received their first cycle of chemotherapy prior to final confirmation of histology because of rapidly progressive disease and deteriorating clinical status. All patients received intrathecal chemotherapy for treatment or prophylaxis. CR was defined using the International Working Group response criteria [Cheson et al. 1999], taking into consideration that many patients did not undergo positron emission tomography (PET) scanning. Those patients who achieved a complete response unconfirmed (CRu) who did not relapse for at least 1 year were considered in retrospect to have achieved a CR. Follow up was per routine clinical care. The Social Security Death Index was queried for patients for whom follow up was remote.
Statistical analyses
Baseline characteristics and treatment characteristics were summarized using descriptive statistics. The CR rates were compared between those who received rituximab versus those who did not using a Pearson chi-square test. The primary outcome was OS, defined as the time from diagnosis to death from any cause. Secondary outcome was EFS, defined as time to relapse, progression, or death from any cause. Kaplan–Meier plots and log-rank tests between those who did versus those did not receive rituximab were performed to compare OS and EFS. Univariable and multivariable models were constructed using Cox proportional hazards regression. All analyses were performed using SAS software, version 9.3 (SAS Institute, Inc.). All tests were two-sided and p values < 0.05 were considered significant.
Results
Patient characteristics
A total of 35 patients were analyzed. Figure 1 shows details of the cohort development and exclusions, resulting in the analysis cohort.
Baseline characteristics of the patients are listed in Table 1. The median age was 44 years. Most patients were white, with Ann Arbor stage IV disease, elevated serum LDH (80.6% greater than the ULN), elevated uric acid level (53.6% greater than the ULN) and preserved performance status (77.8% ECOG PS 0-1). One-third had CNS involvement at diagnosis, and one-half had documented bone marrow involvement.
Table 1.
Baseline characteristics.
| R-Chemo group (N = 18) | Chemo only group (N = 17) | Entire cohort (N = 35) | |
|---|---|---|---|
| Age (median, range) | 42 years (20–70) | 42 years (21–74) | 44 years (20–74) |
| Sex | |||
| Male | 9 (50%) | 11 (64.7%) | 20 (57%) |
| Female | 9 (50%) | 6 (36.3%) | 15 (43%) |
| Race | |||
| White | 15/17 (88.2%)* | 16/17 (94.1%) | 31/34 (91.2%)* |
| Black | 1/17* (5.9%) | 1/17 (5.9%) | 2/34 (5.9%)* |
| Other | 1/17* (5.9%) | 0/17 (0%) | 1/34 (2.9%)* |
| Ann Arbor Stage | |||
| I | 2 (11.1%) | 2 (11.8%) | 4 (11.4%) |
| II | 3 (16.7%) | 0 (0%) | 3 (8.6%) |
| III | 1 (5.6%) | 2 (11.8%) | 3 (8.6%) |
| IV | 12 (66.7%) | 13 (76.5%) | 25 (71.4%) |
| Elevated lactate dehydrogenase | 14/17 (82.4%)* | 11/14 (78.6%)* | 25/31 (80.6%)* |
| Elevated uric acid | 11/17 (61.1%)* | 4/11 (36.4%)* | 15/28 (53.6%)* |
| CNS involvement | 5/18 (27.8%) | 5/12 (41.7%)* | 10/30 (33.3%)* |
| Bone marrow involvement | 8/16 (50%)* | 8/16 (50%)* | 16/32 (50.0%)* |
| Performance status | |||
| 0 | 0/18 (0%) | 2/14 (14.3%)* | 2/32 (6.2%)* |
| 1 | 14/18 (77.8%) | 9/14 (64.3%)* | 23/32 (71.9%)* |
| 2 | 3/18 (16.7%) | 1/14 (7.1%)* | 4/32 (12.5%)* |
| 3 | 1/18 (5.6%) | 1/14 (7.1%)* | 2/32 (6.2%)* |
| 4 | 0/18 | 1/14 (7.1%)* | 1/32 (3.1%)* |
| IPI | |||
| 0 | 2/17 (11.8%)* | 0 (0%) | 2/30 (6.7%)* |
| 1 | 1/17 (5.9%)* | 4/13 (30.8%)* | 5/30 (16.7%)* |
| 2 | 6/17 (35.3%)* | 3/13 (23.1%)* | 9/30 (30.0%)* |
| 3 | 4/17 (23.5%)* | 4/13 (30.8%)* | 8/30 (26.7%)* |
| 4 | 3/17 (17.6%)* | 2/13 (15.4%)* | 5/30 (16.7%)* |
| 5 | 1/17 (5.9%)* | 0 (0%) | 1/30 (3.3%)* |
Denominator reflects missing data.
CNS, central nervous system; IPI, International Prognostic Index.
Treatment regimens
Patients were treated with several different chemotherapeutic regimens, reflecting institutional practice patterns, available clinical protocols, and the introduction of rituximab (see Table 2). HyperCVAD (hyperfractionated cyclophosphamide, vincristine doxorubicin, and dexamethasone alternating with cycles of methotrexate and cytarabine) was the most commonly utilized regimen, with 74.3% of the cohort receiving either HyperCVAD or R-HyperCVAD (rituximab + hyperfractionated cyclophosphamide, vincristine doxorubicin, and dexamethasone alternating with cycles of methotrexate and cytarabine). Overall, 18 (51.4%) participants received rituximab as part of their initial treatment regimen and 17 (48.6%) did not. The median year of diagnosis for patients who received rituximab was 2007 (2001–2008) and for those who did not receive rituximab was 2000 (1998–2004). Eight patients (22.9%) underwent autologous stem cell transplantation (1 as salvage therapy and 7 as part of initial therapy) and 4 (11.4%) underwent allogeneic stem cell transplantation as salvage therapy.
Table 2.
Initial chemotherapeutic regimen.
| Regimen | With rituximab (N = 18) (number, column percentage) | Without rituximab (N = 17) (number, column percent) | Total (N = 35) (number, column percent) |
|---|---|---|---|
| HyperCVAD | 17 (94.4%) | 9 (52.9%) | 26 (74.2%) |
| CODOX-M/IVAC | 1 (5.6%) | 1 (5.9%) | 2 (5.7%) |
| CALGB 9251 | 0 | 7 (41.2%) | 7 (20.0%) |
HyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with cycles of methotrexate and cytarabine; R-HyperCVAD, rituximab + HyperCVAD; CODOX-M-IVAC, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, ara-C.
Responses and survival
Overall, the CR rate was 82.9%. As shown in Table 3, patients who received a rituximab-containing regimen had a higher CR rate than those who did not receive rituximab, although this did not achieve statistical significance (94.4% CR with rituximab versus 70.6% without rituximab, p = 0.088). Among patients who achieved a CR, more than one-quarter (27.6%) relapsed.
Table 3.
Complete response rates following initial therapy.
| Complete response rate |
p value | Relapse after complete response | |||
|---|---|---|---|---|---|
| Overall | With rituximab | Without rituximab | |||
| Any chemotherapy | 29/35 (82.9%) | 17/18 (94.4%) | 12/17 (70.6%) | 0.088 | 8/29 (27.6%) |
| HyperCVAD | 22/26 (84.6%) | 16/17 (94.1%) | 6/9 (66.7%) | 0.104 | 6/22 (27.3%) |
| CODOX-M/IVAC | 2/2 (100%) | 1/1 (100%) | 1/1 (100%) | NA | 0/2 (0%) |
| CALGB 9251 | 5/7 (71.4%) | 0/0 | 5/7 (71.4%) | NA | 2/5 (40%) |
HyperCVAD, hyperfractionated cyclophosphamide, vincristine doxorubicin, and dexamethasone alternating with cycles of methotrexate and cytarabine; R-HyperCVAD, rituximab + HyperCVAD; CODOX-M/IVAC, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, ara-C; CALGB 9251, Cancer and Leukemia Group B multidrug regimem plot study 9251.
The median follow up for the entire cohort was 1.7 years (range 0–12.0) and among survivors was 3.6 years (range 0.9–12.0 years). Figure 2 depicts EFS and OS by use of rituximab. Inclusion of rituximab in the treatment regimen was associated with significantly longer OS (5-year OS 70% and 29%, respectively, p = 0.040) but did not achieve significance for EFS (5-year EFS 61% and 29%, respectively, p = 0.095).
Figure 2.
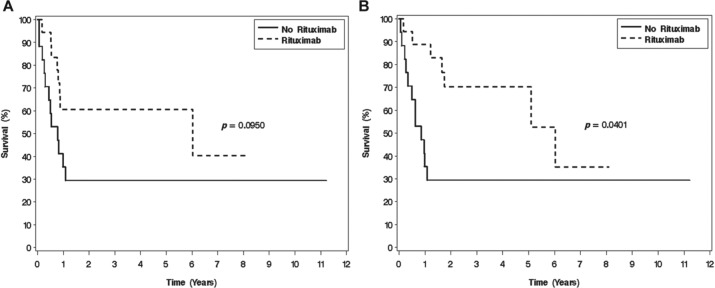
(A) Event-free survival and (B) overall survival for the entire cohort according to whether treatment included rituximab.
Factors predictive of outcome
We performed univariate and multivariate analyses to identify factors associated with inferior survival (Table 4). On univariate analysis, poor performance status, CNS involvement, and not receiving rituximab with chemotherapy were associated with inferior survival. On multivariate analysis, poor performance status or CNS involvement predicted a significantly greater risk of death (adjusted hazard ratio [HR] 15.1, p = 0.001 and 4.55, p = 0.023, respectively). Rituximab was associated with a nearly 70% lower risk of death (adjusted HR 0.32, 95% confidence intervals 0.09–1.18, p = 0.088).
Table 4.
Multivariate analysis of predictors of death.
| Univariate HR | p | Adjusted HR* | p | |
|---|---|---|---|---|
| Age | 1.02 (0.98-1.05) | 0.34 | – | – |
| Race (black/other relative to white | 0.82 (0.11-6.24) | 0.85 | – | – |
| Male gender (relative to female) | 1.55 (0.61-3.97) | 0.36 | – | – |
| Performance status ≥2 | 6.31 (2.09-19.06) | 0.001 | 15.14 (3.31-69.17) | 0.001 |
| Stage IV (relative to stage I–III) | 0.85 (0.30-2.37) | 0.75 | – | – |
| Bone marrow involvement | 0.85 (0.33-2.21) | 0.74 | – | – |
| Central nervous system involvement | 2.70 (0.97-7.49) | 0.06 | 4.55 (1.23-16.79) | 0.023 |
| LDH >ULN | 4.72 (0.62-35.76) | 0.13 | – | – |
| Uric acid >ULN | 1.33 (0.46-3.84) | 0.60 | – | – |
| Chemo (HyperCVAD relative to other BL regimen) | 0.67 (0.22-2.04) | 0.48 | – | – |
| Rituximab | 0.38 (0.15-0.99) | 0.048 | 0.32 (0.09-1.18) | 0.088 |
| IPI (≤ 2 versus ≥3) | 0.66 (0.24-1.83) | 0.43 | – | – |
| Year of diagnosis (2004–2008 versus 1998–2003) | 0.46 (0.18-1.18) | 0.11 |
Adjusted for other predictors in the model.
HR, hazard ratio; LDH, lactate dehtdrogenase; ULN, upper limit of normal; IPI, International Prognostic Index; BL, Burkitt lymphoma; HyperCVAD, hyperfractionated cyclophosphamide, vincristine doxorubicin, and dexamethasone alternating with cycles of methotrexate and cytarabine.
Discussion
This two-institution retrospective cohort study, restricted to cases meeting WHO 2008 criteria for BL, demonstrates a significant OS benefit associated with the addition of rituximab to dose-intense chemotherapeutic regimens for BL. Given that BL is CD20-positive, and extrapolating from the clear benefit of rituximab in DLBCL [Coiffier et al. 2002; Pfreundschuh et al. 2006], it is reasonable to hypothesize that rituximab might improve outcomes in BL. However, the existing literature is unclear in this regard, with three retrospective studies showing no significant improvement in outcomes with the addition of rituximab to dose-intense regimens [Barnes et al. 2011; Todeschini et al. 2012; Wästerlid et al. 2011] and one study showing a clear improvement in response rates, EFS and OS with rituximab among clinical trial participants [Fayad et al. 2007; Thomas et al. 2006]. Table 5 compares the results of these studies along with the current study. The ages of patients across the studies in Table 5 were similar, but the cohorts of patients treated outside of clinical trials [Barnes et al. 2011; Todeschini et al. 2012; Wästerlid et al. 2011] tended to have poorer performance status and higher rates of CNS involvement. This heterogeneity of patients in the cohort studies may account for the failure to detect significant improvements in outcomes with rituximab. One study [Barnes et al. 2011] suggested that there may be a higher rate of infectious complications of therapy with rituximab (although this was not statistically significant), which could plausibly negate the benefit of rituximab if it resulted in higher treatment-related mortality or resulted in decreased dose-intensity.
Table 5.
Studies of addition of rituximab to chemotherapy for Burkitt lymphoma.
| Study | Year | N | Chemotherapy regimen | Age (median, range) | Study type | Diagnoses | Median follow-up | CR rate |
EFS/PFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R-Chemo | Chemo alone | R-Chemo | Chemo alone | p value | R-Chemo | Chemo alone | p value | Multivariate HR for OS (95% confidence intervals) | ||||||||||
| Thomas et al. [2006] | 2006 | 31 | HyperCVAD | 46 (17–77)* | Single-center clinical trial | BL, B-ALL | 22 months | 86% | 85% | 3-year EFS 89% | 3-year EFS 52% | 0.02 | 3-year OS 89% | 3-year OS 53% | < 0.01 | 0.26, p < 0.01 | ||
| Barnes et al. [2011] | 2011 | 80 | CODOX-M/IVAC | 46 (17–78) | Single-center retrospective cohort study | BL | 40.3 months | 90% | 85% | 3-year PFS 74% | 3-year PFS 61% | 0.43 | 3-year OS 77% | 3-year OS 66% | 0.43 | 0.56 (0.23–1.38) | ||
| Wästerlid et al. [2011] | 2011 | 156 | Multiple | 56 (16–93) | Cancer registry study | BL | 41 months | NR | NR | NR | NR | 2-year OS 71.1% | 2-year OS 74.1% | 0.8 (0.5–3.0)† | ||||
| Maruyama et al. [2010] | 2011 | 15 | CODOX-M/IVAC± R | 39 (19–59) | Single-center retrospective cohort study | BL, BLL | 74 months | 8/9 | 5/6 | |||||||||
| Todeschini et al. [2012] | 2012 | 71 | POG 8617 | NR (3–77) | 4 center retrospective cohort study | BL | NR | NR | NR | 69.6% | 73.9% | NS | NR | NR | NR | NR | ||
| Dujmovic et al. [2012] | 2012 | 20 | B-NHL 86 | 35 (16–63) | Single-center retrospective cohort study | BL | 39 months | 83% | 38% | 83% | 38% | 0.039 | 83% | 38% | 0.039 | NR | ||
| Ribrag et al. [2012] | 2012 | 257 | COPADM | 47 | Phase III trial | BL | 38 months | 3-year EFS 76% | 3-year EFS 64% | 0.046 | 3-year OS 82% | 3-year OS 71% | 0.016 | |||||
| Wästerlid et al. [2013] | 2013 | 258 | Multiple | 56 (15–93) | Cancer registry study | BL | NR | NR | NR | NR | 2-year OS | 2-year OS | 0.98 (0.57–1.72) | |||||
| R-BFM | 83.7% | BFM | 81.8% | |||||||||||||||
| R-CODOX- | 71.4% | CODOX- | 66.7% | |||||||||||||||
| M/IVAC | M/IVAC | |||||||||||||||||
| R-CHOP/ | 35% | CHOP/ | 33.3% | |||||||||||||||
| CHOEP | CHOEP | |||||||||||||||||
| Present study | 2013 | 35 | Multiple | 44 (20–74) | Two-center retrospective cohort study | BL | 1.6 years | 94.4% | 70.6% | 5-year EFS 60.6% | 5-year EFS 29.4% | 0.095 | 5-year OS 70.2% | 5-year OS 29.4% | 0.040 | 0.32 (0.09–1.18) | ||
R-HyperCVAD group.
Univariate.
CR, complete response; EFS, event-free survival; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; NR, not reported; NS, not significant; BL, Burkitt lymphoma; B-ALL, acute lymphoblastic lymphoma; BLL, Burkitt-like lymphoma (B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and BL); DLBCL, diffuse large B-cell lymphoma with > 95% proliferation index); HyperCVAD, hyperfractionated cyclophosphamide, vincristine doxorubicin, and dexamethasone alternating with cycles of methotrexate and cytarabine; R-HyperCVAD, rituximab + HyperCVAD; R-HyperCVAD, rituximab + hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone); CODOX-M/IVAC, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, ara-C; COPADM, cyclophosphamide, vincristine, prednisone, adriamycin, high-dose methotrexate; R-CODOX (Rituximab+CODOX); CHOEP, cyclophosphamide, doxorubicin, vincristine etoposide, prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
It is unlikely that a prospective randomized trial will be completed to rigorously define the role of rituximab in BL. As a result, based on our results and others, and given the favorable toxicity profile of rituximab when added to dose-intensive regimens, we recommend the combination of rituximab with dose-intensive chemotherapy regimens in patients who are candidates for aggressive therapy.
Our study also supports the previously described prognostic significance of poor performance status [Thomas et al. 1999; Wästerlid et al. 2011] and CNS involvement [Barnes et al. 2011; Thomas et al. 2006]. On multivariate analysis, performance status remained strongly predictive of death; patients having an ECOG performance status of 2 or greater had a 15-fold greater risk of death than those with a performance status of 0–1. Rituximab nearly retained significance in the multivariate analysis with a threefold greater risk of death in patients not treated with rituximab (p = 0.088). CNS involvement retained prognostic significance on multivariate analysis. This multivariate analysis was performed among patients who received dose-intense chemotherapy. Thus, inferior outcomes are unlikely to simply reflect inadequate initial therapy; however, data on toxicity of therapy and dose-intensity were not captured in this retrospective study. It is possible that poor performance status could have been associated with increased toxicity or decreased dose density, resulting in poorer survival. Our study did not find a statistically significant impact of advancing age on survival among patients treated with dose-intense chemotherapy regimens. This is similar to one recent study [Todeschini et al. 2012], but contrasts with several other studies, including a systematic review [Kelly et al. 2009] and two recent SEER registry studies [Castillo et al. 2013; Costa et al. 2013] which found poorer outcomes with advancing age. In the current study, only patients who received dose-intense chemotherapeutic regimens were included; thus, there was no age-associated difference in survival among patients deemed fit to receive dose-intense chemotherapy.
Strengths of this study include the rigorous definition of BL using modern hematopathology techniques and the inclusion of all patients receiving modern chemotherapy, not just those eligible for a clinical trial. The 5-year OS rate of our entire cohort was 50.3%; this is lower than the 5-year OS rate seen in clinical trial populations, but is similar to that described in studies of the SEER registry (46% in the 1997–2007 time period [Castillo et al. 2013] and 56% in the 2002–2008 time period [Costa et al. 2013]). Our results reflect the outcomes of patients treated largely outside of clinical trials at two institutions, including over 10% of the cohort who had a WHO performance status of 3–4. In a Swedish population-based study, the proportion of patients with WHO performance status 3–4 was 21.2% [Wästerlid et al. 2011], compared with the clinical trial population who received R-HyperCVAD where 0% of patients had an ECOG performance status of 3–4 [Thomas et al. 2006]. Thus, even though our cohort was restricted to those who had received dose-intense chemotherapy regimens, it contained a heterogeneity of patients similar to that seen on the population level, highlighting how select a group clinical trial participants are. This is an issue of great importance for a disease such as BL, in which performance status at diagnosis has a profound impact on outcome.
Limitations of this retrospective analysis include possible imbalances in the treatment groups, which could account for the differences in outcomes. The differences in OS between those who did versus those who did not receive rituximab could be confounded by changes in supportive care over time, as the shift in the use of rituximab coincides with improvements in supportive care. However, year of diagnosis (as a proxy for changes in supportive care) was not significantly associated with survival on univariate analysis, nor when it was forced into the multivariate model. Differences in the chemotherapy regimen between the groups who did versus the groups who did not receive rituximab could potentially confound the association between rituximab and OS. However, chemotherapy regimen was not associated with survival (Table 4), although the number of patients in each chemotherapy group was small. The proportion of patients in our study (33.3%) who had CNS involvement was greater than is typically reported (8.5–19% in cohort studies) [Barnes et al. 2011; Wästerlid et al. 2013] and may suggest a referral bias of higher-risk patients to the two academic medical centers. Propensity score analysis [Rosenbaum and Rubin, 1983] can be informative for controlling for confounders, but is not feasible with our relatively small sample size.
In summary, in a cohort of patients with BL confirmed by rigorous hematopathology techniques, treated at two academic medical centers, the inclusion of rituximab in dose-intense chemotherapeutic regimens was associated with a significant improvement in OS. Multivariate modeling confirmed the prognostic significance of poor performance status and CNS involvement, with receipt of rituximab nearly retaining significance. Our data indicate that for BL patients who are candidates for aggressive therapy, enhanced outcomes are achieved when rituximab is combined with a dose-intensive regimen. However, in this unselected population, the 5-year EFS is only 60%, underscoring that new treatment approaches are still needed for this patient population.
Footnotes
Funding: This work was supported by the National Cancer Institute (NCI) at the National Institutes of Health (NIH) (grant numbers K12CA167540 and KM1CA156708) and The Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (grant numbers UL1 RR024992, KL2 RR024994, and TL1 RR024995) and the National Center for Advancing Translational Sciences at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NCATS or NIH. This work was also supported in part by the Foundation for Barnes-Jewish Hospital and its generous donors (to NLB and KVF).
Conflict of interest statement: The authors have no financial conflicts of interest to disclose.
Contributor Information
Tanya M. Wildes, Division of Medical Oncology, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8056, St Louis, MO 63110, USA
Laura Farrington, Division of Hematology and Oncology, East Tennessee State University College of Medicine, Johnson City, TN, USA.
Cecilia Yeung, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Alexandra M. Harrington, Department of Pathology, Medical College of Wisconsin, Milwaukee, WI, USA
Kelley V. Foyil, Division of Medical Oncology, Washington University School of Medicine, St Louis, MO, USA
Jingxia Liu, Division of Biostatistics, Washington University School of Medicine, St Louis, MO, USA.
Friederike Kreisel, Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO, USA.
Nancy L. Bartlett, Division of Medical Oncology, Washington University School of Medicine, St Louis, MO, USA
Timothy S. Fenske, Medical College of Wisconsin, Division of Hematology & Oncology, Milwaukee, WI, USA
References
- Barnes J., Lacasce A., Feng Y., Toomey C., Neuberg D., Michaelson J., et al. (2011) Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol 22: 1859–1864 [DOI] [PubMed] [Google Scholar]
- Bennett J., Catovsky D., Daniel M., Flandrin G., Galton D., Gralnick H., et al. (1985) Proposed revised criteria for the classification of acute myeloid leukemia A report of the French-American-British Cooperative Group. Ann Int Med 103: 620–625 [DOI] [PubMed] [Google Scholar]
- Blayney D., LeBlanc M., Grogan T., Gaynor E., Chapman R., Spiridonidis C., et al. (2003) Dose-intense chemotherapy every 2 weeks with dose-intense cyclophosphamide, doxorubicin, vincristine, and prednisone May improve survival in intermediate- and high-grade lymphoma: a phase II Study of the Southwest Oncology Group (SWOG 9349). J Clin Oncol 21: 2466–2473 [DOI] [PubMed] [Google Scholar]
- Castillo J., Winer E., Olszewski A. (2013) Population-based prognostic factors for survival in patients with Burkitt lymphoma: An analysis from the Surveillance, Epidemiology, and End Results database. Cancer 119: 3672–3679 [DOI] [PubMed] [Google Scholar]
- Cheson B., Horning S., Coiffier B., Shipp M., Fisher R., Connors J., et al. (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17: 1244–1253 [DOI] [PubMed] [Google Scholar]
- Coiffier B., Lepage E., Brière J., Herbrecht R., Tilly H., Bouabdallah R., et al. (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346: 235–242 [DOI] [PubMed] [Google Scholar]
- Costa L., Xavier A., Wahlquist A., Hill E. (2013) Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: an analysis of 3691 cases. Blood 121: 4861–4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave S., Fu K., Wright G., Lam L., Kluin P., Boerma E., et al. (2006) Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med 354: 2431–2442 [DOI] [PubMed] [Google Scholar]
- Dujmovic D., Aurer I., Radman I., Serventi-Seiwerth R., Dotlic S., Stern-Padovan R., et al. (2012) Addition of rituximab to high-dose methotrexate-based chemotherapy improves survival of adults with Burkitt lymphoma/leukemia. Acta Haematologica 127: 115–117 [DOI] [PubMed] [Google Scholar]
- Dunleavy K., Pittaluga S., Wayne A., Shovlin M., Johnson J., Little R., et al. (2011) Myc+ aggressive B-cell lymphomas: novel therapy of untreated Burkitt lymphoma (BL) and Myc+ diffuse large B-cell lymphoma (DLBCL) with DA-EPOCH-R. Ann Oncol 22(54): 71 [Google Scholar]
- Evens A., Carson K., Kolesar J., Nabhan C., Helenowski I., Islam N., et al. (2013) A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt’s lymphoma. Ann Oncol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad L., Thomas D., Romaguera J. (2007) Update of the M. D. Anderson Cancer Center experience with hyper-CVAD and rituximab for the treatment of mantle cell and Burkitt-type lymphomas. Clin Lymph Myeloma 8(Suppl. 2): S57–S62 [DOI] [PubMed] [Google Scholar]
- Harris N., Jaffe E., Diebold J., Flandrin G., Müller-Hermelink H., Vardiman J., et al. (1999) The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol 10: 1419–1432 [DOI] [PubMed] [Google Scholar]
- Hummel M., Bentink S., Berger H., Klapper W., Wessendorf S., Barth T., et al. (2006) A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 354: 2419–2430 [DOI] [PubMed] [Google Scholar]
- Kantarjian H., Walters R., Keating M., Smith T., O’Brien S., Estey E., et al. (1990) Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol 8: 994–1004 [DOI] [PubMed] [Google Scholar]
- Kelly J., Toothaker S., Ciminello L., Hoelzer D., Holte H., LaCasce A., et al. (2009) Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymph Myeloma 9: 307–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama D., Watanabe T., Maeshima A., Nomoto J., Taniguchi H., Azuma T., et al. (2010) Modified cyclophosphamide, vincristine, doxorubicin, and methotrexate (CODOX-M)/ifosfamide, etoposide, and cytarabine (IVAC) therapy with or without rituximab in Japanese adult patients with Burkitt lymphoma (BL) and B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and BL. Int J Hematol 92: 732–743 [DOI] [PubMed] [Google Scholar]
- Mead G., Sydes M., Walewski J., Grigg A., Hatton C., Norbert P., et al. (2002) An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: Results of United Kingdom Lymphoma Group LY06 study. Ann Oncol 13: 1264–1274 [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M., Trümper L., Österborg A., Pettengell R., Trneny M., Imrie K., et al. (2006). CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7: 379–391 [DOI] [PubMed] [Google Scholar]
- Ribrag V., Koscielny S., Bouabdallah K., Salles G., Casasnovas O., Recher C., et al. (2012) Addition of rituximab improves outcome of HIV negative patients with Burkitt lymphoma treated with the LMBA protocol: results of the Randomized Intergroup (GRAALL-Lysa) LMBA02 Protocol. Blood (ASH Annual Meeting Abstracts) 120: 685a [Google Scholar]
- Rizzieri D., Johnson J., Niedzwiecki D., Lee E., Vardiman J., Powell B., et al. (2004) Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer 100: 1438–1448 [DOI] [PubMed] [Google Scholar]
- Rosenbaum P., Rubin D. (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–55 [Google Scholar]
- Swerdlow S., Campo E., Harris N., Jaffe E., Pileri S., Stein H., et al. (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer [Google Scholar]
- The Non-Hodgkin’s Lymphoma Classification Project (1982) National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. Cancer 49: 2112–2135 [DOI] [PubMed] [Google Scholar]
- Thomas D., Cortes J., O’Brien S., Pierce S., Faderl S., Albitar M., et al. (1999) Hyper-CVAD program in Burkitt’s-type adult acute lymphoblastic leukemia. J Clin Oncol 17: 2461–2470 [DOI] [PubMed] [Google Scholar]
- Thomas D., Faderl S., O’Brien S., Bueso-Ramos C., Cortes J., Garcia-Manero G., et al. (2006) Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 106: 1569–1580 [DOI] [PubMed] [Google Scholar]
- Todeschini G., Bonifacio M., Tecchio C., Balter R., Carli G., Stefani P., et al. (2012) Intensive short-term chemotherapy regimen induces high remission rate (over 90%) and event-free survival both in children and adult patients with advanced sporadic Burkitt lymphoma/leukemia. Am J Hematol 87: 22–25 [DOI] [PubMed] [Google Scholar]
- Wästerlid T., Brown P., Hagberg O., Hagberg H., Pedersen L., D’Amore F., et al. (2013) Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Ann Oncol 24: 1879–1886 [DOI] [PubMed] [Google Scholar]
- Wästerlid T., Jonsson B., Hagberg H., Jerkeman M. (2011) Population based study of prognostic factors and treatment in adult Burkitt lymphoma: a Swedish Lymphoma Registry study. Leuk Lymph 52: 2090–2096 [DOI] [PubMed] [Google Scholar]