Abstract
Myelodysplastic syndrome (MDS) is a heterogenous group of stem cell disorders usually characterized by progressive refractory cytopenias, which could progress to acute myeloid leukemia. MDS may be associated with a wide spectrum of skin lesions, including neoplastic cell infiltration, Sweet's syndrome, pyoderma gangrenosum, erythema elevatum diutinum, vasculitis, and panniculitis. However, erythema nodosum is rarely associated with MDS. Unusual rheumatologic manifestations in patients with MDS also have been reported, which range from asymptomatic serological abnormalities to classic connective tissue disorders such as Sjogren's syndrome, relapsing polychondritis, systemic lupus erythematosus, rheumatoid arthritis and mixed connective tissue disease. However, concurrent erythema nodosum and serositis has rarely been reported. We describe a case of MDS with erythema nodosum and immune-mediated pericardial effusion in a 34-year-old woman.
Keywords: Myelodysplastic syndrome, Erythema nodosum, Pericardial effusion
INTRODUCTION
The myelodysplastic syndrome (MDS) is a heterogenous group of stem cell disorders usually characterized by progressive refractory cytopenias with possible progression to acute myeloid leukemia. Although cutaneous manifestation in MDS is not frequent, MDS may be associated with a wide spectrum of skin lesions1) and unusual rheumatologic manifestations2,3). However, concurrent erythema nodosum and serositis has rarely been reported. We describe a case of MDS with erythema nodosum and immune-mediated pericardial effusion.
CASE REPORT
A 34-year-old woman was diagnosed with MDS French-American-British (FAB) subtype refractory anemia in March, 2000. The patient did not receive any special treatment except intermittent transfusion of packed red cell.
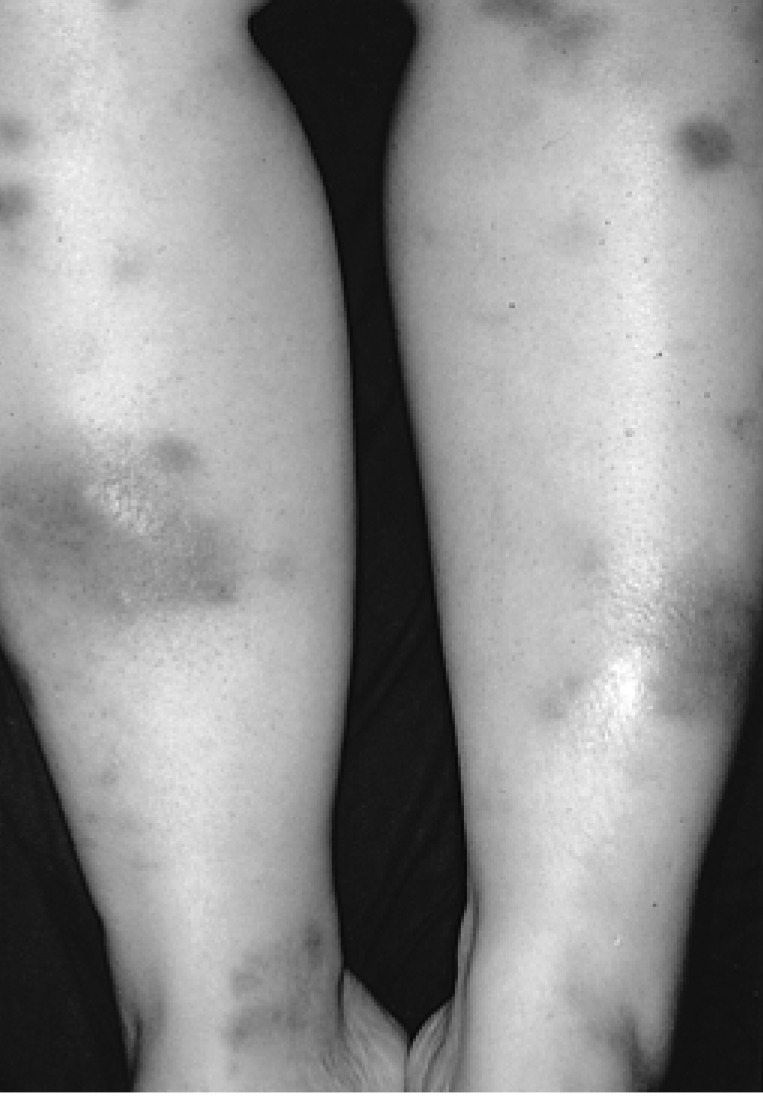
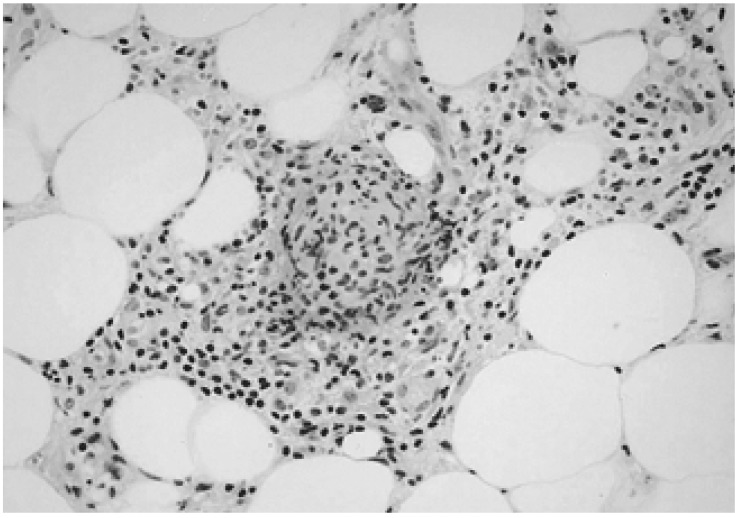
In May, 2003 the patient presented with fatigue, myalgia, fever as well as multiple edematous, tender, erythematous to brownish subcutaneous nodular lesions on both upper and lower extremities and trunk (Figure 1). Laboratory finding indicated hemoglobin 5.8 g/dL, white blood cell count 6.8×109/L, platelet 4.8×1010/L, and normal liver and kidney function test. Biopsy of skin lesion revealed lobular panniculitis consistent with erythema nodosum (Figure 2). The skin lesion had improved with 20 mg of prednisone per day.
Figure 1.
Multiple edematous, tender, erythematous nodules on the extensor surfaces of the legs.
Figure 2.
Histological examination showed lympho-histiocytic infiltration in the septa and the periphery of the fat lobules consistent with lobular panniculitis (H&E stain, ×400).
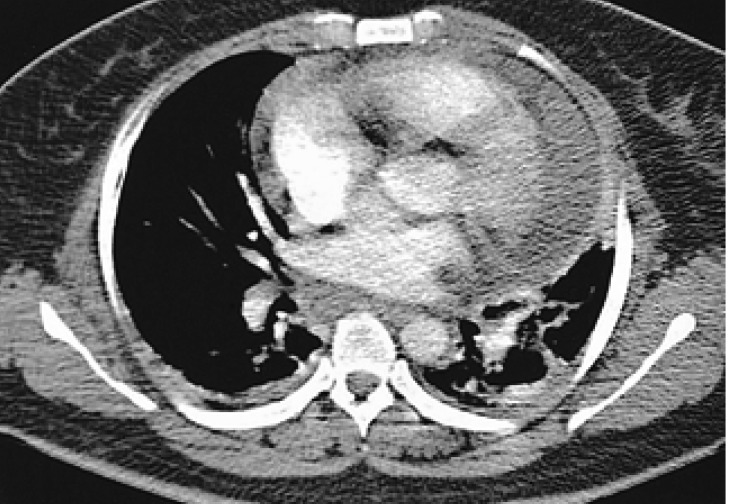
One month later, dyspnea, chest pain and fever developed and the skin lesions were aggravated. She was taking a tapering dose of prednisone (10 mg). The laboratory tests showed hemoglobin 6.0 g/dL, white blood cell count 4.8×109/L, platelet 8.0×109/L, uric acid 2.3 mg/dL, LDH 474 U/L (normal 211-423), serum ferritin 1831 ng/mL, serum iron/TIBC 145/162 µ g/dL. Antinuclear antibody, rheumatoid factor and ANCA were all negative. The levels of C3 and C4 were normal. Blood and urine culture were sterile. Chest X-ray and chest CT scan showed cardiomegaly with pulmonary edema (Figure 3). Echocardiography revealed normal ejection fraction with large amounts of pericardial effusion. After the dose of prednisone was increased to 40 mg/day, the pericardial effusion and skin lesions had remarkably improved, suggesting that these newly developed symptoms might be attributed to the immune-mediated complications of MDS. The repeated bone marrow biopsy performed one month later revealed no evidence of progression to acute leukemia. However, the dysplastic changes in the myeloid, erythroid and megakaryocytes lineages had progressed compared to the examination in the prior month. While gradually tapering prednisone until July 2004, she was taking 5 mg/day of prednisone without pericardial effusion. Platelet count slowly increased and maintained above 2.0×1010/L.
Figure 3.
Chest CT showed pericardial effusion with pulmonary edema and atelectasis.
DISCUSSION
MDS can be associated with a wide spectrum of skin lesions including neoplastic cell infiltration, Sweet's syndrome, pyoderma gangrenosum, erythema elevatum diutinum, vasculitis, and panniculitis1). However, MDS-associated erythema nodosum is relatively rare4). Erythema nodosum is the most frequent clinico-pathological variant of panniculitis and manifests as a cutaneous reaction consisting of inflammatory, tender, nodular lesions, usually located symmetrically on the extensor surfaces of the lower extremities5). Manifestation of the lesions may be associated with a wide variety of conditions including infections, sarcoidosis, rheumatologic diseases, inflammatory bowel diseases, medications, autoimmune disorders, pregnancy and malignancies6-8). The appearance of skin lesions in MDS may herald its progression to acute myeloid leukemia. Although in this case the repeated bone marrow biopsy did not show any findings of progression to acute leukemia, the dysplastic changes in all lineages of hematopoietic cells had progressed with the development of skin lesions.
It has been reported that autoimmune manifestations in patients with MDS range from asymptomatic serological abnormalities to classic connective tissue disorders such as Sjögren's syndrome, relapsing polychondritis, systemic lupus erythematosus, rheumatoid arthritis and mixed connective tissue disease2,3,9).
The pathogenesis of autoimmune features in MDS has not been determined. Several reports describe the dysregulation of cellular and humoral immunity. In particular, abnormal T-cell responses to antigen presentation and abnormal B-cell and T-cell interactions in MDS may be important in the pathogenesis of immune dysregulation leading to the development of an autoimmune phenomenon10-12). Alternatively, autoimmune manifestations may precede the onset of MDS. Thus, abnormalities in T-cell function might be associated with the development of MDS13). Although pericardiocentesis was not performed in our case, the pericardial effusion rapidly regressed after treatment with high doses of systemic corticosteroid, suggesting that this lesion might be a reactive inflammatory change involving immunologic mechanisms.
Enright et al.9) reported that aggressive therapy with immunosuppressive agents in selected patients often controls autoimmune features associated with MDS and may lead to hematological responses in some patients. The most commonly used immunosuppressive agents are oral prednisone and high-dose intravenous methylprednisolone. In addition, some patients received azathioprine, cyclophosphamide, or other agents. Although there may be poor prognosis with the onset of autoimmune disease in patients with MDS, patients with a hematologic response to immunosuppressive therapy have prolonged survival compared with patient who do not. In this case, we noted that treatment with prednisone improved not only erythema nodosum and pericardial effusion, which may be related to an autoimmune manifestation, but also thrombo-cytopenia.
In summary, MDS may be associated with erythema nodosum with skin lesions that may be a result of autoimmune dysfunction. The understanding of the pathophysiology of MDS may contribute to explain the autoimmune manifestations and skin lesions in MDS.
References
- 1.Avivi I, Rosenbaum H, Levy Y, Rowe J. Myelodysplastic syndrome and associated skin lesions: a review of the literature. Leuk Res. 1999;23:323–330. doi: 10.1016/s0145-2126(98)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Castro M, Conn DL, Su WP, Garton JP. Rheumatic manifestations in myelody-splastic syndromes. J Rheumatol. 1991;18:721–727. [PubMed] [Google Scholar]
- 3.Kuzmich PV, Ecker GA, Karsh J. Rheumatic manifestations in patients with myelodysplastic and myeloproliferative diseases. J Rheumatol. 1994;21:1649–1654. [PubMed] [Google Scholar]
- 4.Nishie W, Kimura T, Kanagawa M. Sweet's syndrome evolved from recurrent erythema nodosum in a patient with myelodysplastic syndrome. J Dermatol. 2002;29:91–95. doi: 10.1111/j.1346-8138.2002.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 5.Ryan TJ. Cutaneous vasculitis: erythema nodosum. In: Rook A, Wilkinson DS, Ebling FJ, editors. Textbook of dermatology. Oxford: Blackwel; 1998. pp. 2196–2202. [Google Scholar]
- 6.Bohn S, Buchner S, Itin P. Erythema nodosum: 112 cases: epidemiology, clinical aspects and histopathology. Schweiz Med Wochenschr. 1997;127:1168–1176. [PubMed] [Google Scholar]
- 7.Cribier B, Caille A, Heid E, Grosshans E. Erythema nodosum and associated diseases: a study of 129 cases. Int J Dermatol. 1998;37:667–672. doi: 10.1046/j.1365-4362.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonci A, di Lernia V, Merli F, Lo Scocco G. Erythema nodosum and Hodgkin's disease. Clin Exp Dermatol. 2001;26:408–411. doi: 10.1046/j.1365-2230.2001.00847.x. [DOI] [PubMed] [Google Scholar]
- 9.Enright H, Jacob HS, Vercellotti G, Howe R, Belzer M, Miller W. Paraneoplastic autoimmune phenomena in patients with myelodysplastic syndromes: response to immunosuppressive therapy. Br J Haematol. 1995;91:403–408. doi: 10.1111/j.1365-2141.1995.tb05310.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida Y. Biology of myelodysplastic syndromes. Int J Cell Cloning. 1987;5:356–375. doi: 10.1002/stem.5530050502. [DOI] [PubMed] [Google Scholar]
- 11.Mufti GJ, Figes A, Hamblin TJ, Oscier DG, Copplestone JA. Immunological abnormalities in myelodysplastic syndromes: I. serum immunoglobulins and autoantibodies. Br J Haematol. 1986;63:143–147. doi: 10.1111/j.1365-2141.1986.tb07504.x. [DOI] [PubMed] [Google Scholar]
- 12.Colombat PH, Renoux M, Lamagnere JP, Renoux G. Immunologic indices in myelodysplastic syndromes. Cancer. 1988;61:1075–1081. doi: 10.1002/1097-0142(19880315)61:6<1075::aid-cncr2820610604>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Karcher DS, Frost AR. The bone marrow in human immunodeficiency virus (HIV)-related disease: morphology and clinical correlation. Am J Clin Pathol. 1991;95:63–71. doi: 10.1093/ajcp/95.1.63. [DOI] [PubMed] [Google Scholar]