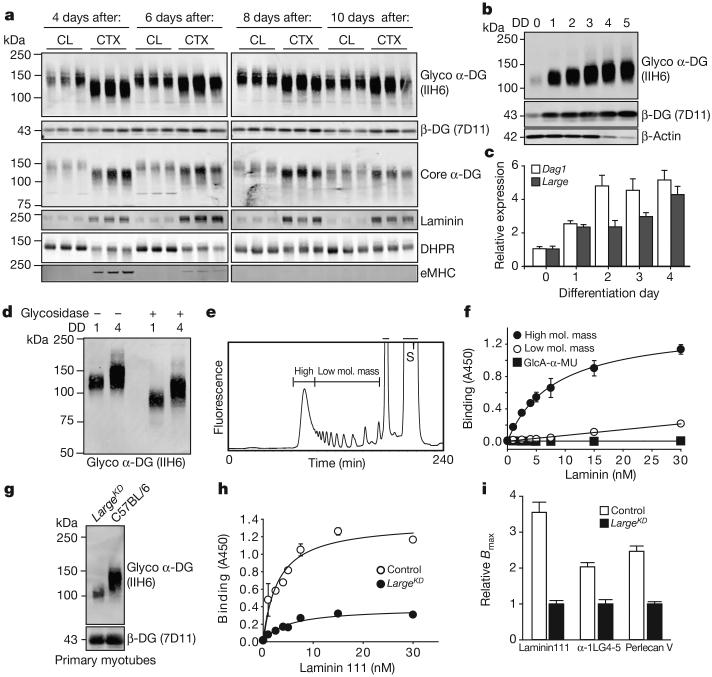
Figure 3. Increase in α-DG LARGE-glycan chain length during myogenesis enhances ligand-binding capacity.
a, Western blot analysis of WGA-enriched samples for expression of DG and other proteins at multiple times after CTX-mediated injury to C57BL6/J mice, each lane representing an individual animal. b, Western blot analysis of WGA-enriched lysates from C2C12 myoblasts undergoing myogenesis for functional α-DG (DD, differentiation day). At DD4–5 the loading control, β-actin, is downregulated in myotubes. c, qPCR analysis of Dag1 and Large during C2C12 myogenesis (averages reported, P<0.001, n=3 biological replicates, n≥7 technical replicates, relative to Rpl4 expression, error bars indicate s.e.m.). d, Western blot demonstrating effects of enzymatic deglycosylation of DD0 and DD4 C2C12 protein samples. e, f, Elution profile of LARGE-glycan repeats on a gel filtration column. Bars indicate the fractions that were collected as the high- and low-molecular-mass LARGE repeats (S, substrate) used for solid-phase laminin 111 (f, high molecular mass Kd=8.2) binding. g, DG western blot in DD4 myotubes from LargeKD (+1 μgml−1 doxycycline) and control cultures. h, Solid-phase laminin 111 binding demonstrating that binding capacity is dependent on extension of the LARGE-glycan (control Kd=2.93 ± 0.776 nM; LargeKD Kd=4.75 ± 1.24 nM). i, Comparison of solid-phase determined relative Bmax values for several α-DG ligands (averages represented, Bmax values for LargeKD were set to 1 to allow for direct comparisons; error bars indicate s.e.m.). For solid-phase assays (f and h), error bars indicate s.e.m., n=3 technical replicates, curve fitting to equation f=Bmax*abs(x)/(Kd + abs(x)).