Abstract
Adenoviral transduction of human bladder cancer cells with human interferon α-2b (Ad-IFN) produces cancer specific cell death via direct and indirect mechanisms. The indirect mechanisms involve the secreted IFN produced which kill IFN protein sensitive cancer cells as well as yet unidentified bystander factors which are cytotoxic to neighboring cancer cells. The direct cell kill results from transfection and expression of Ad-IFN in the cancer cells. Since the molecular forms of cytokeratin 18 (CK18), either caspase cleaved or not, have been associated with apoptotic or necrotic cell death, respectively, we determined if increases in either or both CK18 forms could be observed following IFNα protein or Ad-IFN treatment of bladder carcinoma cells. Quantification of M30 and M65 ELISAs (assays for CK18 associated apoptotic and necrotic cell death, respectively) were used as surrogate markers of the cell death produced. In the IFN protein sensitive RT4 bladder cancer cells IFN produced primarily M30 related cell death whereas Ad-IFN treatment resulted in high levels of both M30 and M65. In contrast, conditioned medium from Ad-IFN treated cells whether from normal human urothelial cells or bladder cancer cells caused mainly increases in M30 levels when added to IFN protein resistant KU7 or UC9 bladder cancer cells, suggesting that the bystander factors present in the CM produced primarily apoptotic cell death. In addition, a significant increase in M65 levels above that observed for M30 was seen when the IFN protein resistant KU7 and UC9 cells were treated with Ad-IFN, again indicating there is additional necrotic related cell death produced by Ad-IFN as well. Normal urothelial cells showed no cytotoxicity nor increases in M30 or M65 following Ad-IFN treatment. Since intravesical Ad-IFN treatment is presently being evaluated for its efficacy in superficial bladder cancer that measurement of M30 and M65 levels in the urine at various time-points before and after Ad-IFN treatment may provide not only a biomarker of efficacy but also evidence for the different types and proportion of cell kill produced by the various mechanisms of cell kill in the tumors of individual patients.
Keywords: Adenoviral-mediated interferon α cell death, extracellular cytokeratin 18, M30 and M65 ELISAs, bladder cancer
INTRODUCTION
Cytotoxicity produced by the human interferon α-2b (Intron A) in bladder cancer cells is mediated largely through an apoptotic mechanism involving the autocrine production of TRAIL in those cells sensitive to the IFNα protein (1). However, many bladder cell lines are highly resistant to IFNα (1). These IFNα resistant cells, however, proved to be highly sensitive to Ad-IFN when given in vivo as an intravesical instillation or in cell culture (2). This indicated that Ad-IFN treatment had other mechanisms to cause cytotoxicity in addition to the production of recombinant IFNα. Further evidence for this belief was provided by the finding that Ad-IFN had a strong bystander effect in these IFN resistant cells (2). The bystander factor(s) were later found to be present in conditioned medium following Ad-IFN treatment of both normal urothelial or various cancer cells resistant to IFNα, although normal cells showed no cytotoxicity to Ad-IFN (3, 4). In addition, the Ad-IFN induced bystander effect was mediated by the soluble factor(s) to induce apoptosis by TRAIL - and caspase 8-independent mechanisms (3, 4).
An intravesical Phase l study using Ad-IFN is now underway for superficial bladder cancer based in large part on our preclinical results (2). We thought it would be most helpful in this trial not only to document the levels of IFN achieved in the urine at various concentrations of Ad-IFN instillation but also to try to identify possible biomarkers of Ad-IFN produced bladder cancer cell death. The quantification of M30 and M65 ELISAs as surrogate biomarkers of cell death appear to be reasonable candidates. These ELISAs detect different forms of the epithelial cell structural protein cytokeratin 18 (CK 18) and have been proposed as surrogate assays of tumor cell death (5, 6). The M30 antibody recognizes a CK18 fragment that is produced following caspase cleavage of the CK18 protein and is postulated to be a selective biomarker of apoptotic cell death, whereas the M65 ELISA uses the M5 antibody thought to detect the uncleaved as well as cleaved CK18 protein fragment. Therefore the M65 ELISA measures intact CK18 protein indicative necrotic cell death as well as the cleaved CK18 form involved in apoptotic cell death. By subtracting the concentration of cleaved CK18 obtained from the M30 ELISA, considered to be the apoptotic component, from the level obtained by the M65 ELISA, a measure of the necrotic component of cell death is theoretically determined.
Both ELISAs are being used at present in the pharmacodynamic evaluation of changes in the plasma following anticancer treatment (7, 8). In turn these assays could be ideal to document possible apoptotic and/or necrotic components in the urine at different time points following intravesical Ad-IFN treatment. In this report the changes in M30 and M65 levels were examined in the supernatant of IFN protein sensitive and resistant bladder cancer cell lines and normal human urothelium following IFNα or Ad-IFN exposure. In addition, Ad-IFN-produced conditioned medium from either IFN resistant cancer or normal urothelium was examined on IFN resistant cancer cells to evaluate M30 and M65 changes produced by bystander factor(s).
We hypothesized that the cell death produced by Intron A in IFN sensitive cancer cells would be primarily apoptotic which would result in increases in M30 but not M65 levels, whereas the direct effect of Ad-IFN on cancer or normal urothelial cells was unknown. We also believed that the information obtained on M30 and M65 levels in the supernatant from various treated cells will be reflected in the urine from patients treated with intravesical Ad-IFN in the ongoing superficial bladder cancer study.
MATERIALS AND METHODS
Cell lines
The bladder cancer cell line, UM-UC9, was provided by Dr. Barton Grossman and bladder cancer cell lines, KU7 and RT4, were available in our laboratory. The UC9, KU7 and RT4 cells were grown in MEM, RPMI 1640 and McCoys 5A medium, respectively in 10% with fetal bovine serum and incubated at 37°C in 5% CO2 and 95% air. The normal urothelial cell line (TERT-NHUC) was provided by Dr. Margaret Knowles and grown in K-SFM medium with cholera toxin (9).
Reagents and Ad-IFN or Intron A treatment
Ad-IFN α2b (Ad-IFN) and Ad-β-gal were obtained from the Schering-Plough Research Institute (Kenilworth, NJ). Cells were exposed to the adenoviral vectors at a concentration of 1×107 or 2×107 pfu/ml (a 50 or 100 MOI, respectively) for 3h in medium without serum. The virus was then removed and complete control medium added. Recombinant interferon α-2b (Intron A, Schering-Plough) was purchased from the MD Anderson Pharmacy. The bladder cancer and TERT-NHU cells were seeded into 6 well plates at 1×105 cells/per well and cultured overnight. For the RT4 studies 1000, 3000, or 10,000 IU/ml of IFNα was added to medium containing 10% FBS for 24h. The Intron A was then removed and regular medium added. At different time points, the medium was collected, centrifuged at 1000 rpm in 4°C for 10 minutes, aliquoted at 1ml/per vial and stored at −80°C for future assays. Staurosporine (STS). was purchased from Sigma (St Louis, MO), dissolved in DMSO at 200 μM/ml as stock solution and stored at −20°C. The STS stock solution was diluted to 500nM/ml with medium, added to the cultured cells and incubated at 37°C in 5% CO2 for 3hr. After the 3 hr incubation the STS medium was removed, the cells were washed once with fresh medium and refed with medium containing 10% FBS. At 24h, 48h, and 72hr the medium was centrifuged as given above and stored at −80°C.
Generation of conditioned medium (CM)
The UC9 and KU7 bladder cancer and TERT-NHU cells were plated into 100-mm dishes at 1×106 cells/dish. After Ad-IFN infection as outlined above, the medium was harvested from the Ad-IFN infected dishes at various times after treatment, filtered through a 0.22μm filter and were then termed either UC9-AdIFN-CM, KU7-AdIFN-CM or NHU-AdIFN-CM. The medium harvested from the non-infected control cells were termed UC9-control-CM or KU7-control-CM. All of the conditioned medium were then aliquoted and stored at −80°C.
ELISA M30 and M65 assays
M30-Apoptosense and M65 ELISA kits were obtained from Peviva AB (Bromma, Sweden,) and used according to manufacturer protocol. Briefly, 25μl of each cell culture supernatant and standards were tested in duplicate or triplicate. 75μl of the combined 0.4ml monoclonal M30 antibody (anti-CK18Asp 396 neoepitope) conjugated with horseradish peroxidase (HRP) substrate and the conjugate dilution buffer was added to each well for the M30-Apoptosense ELISA and 0.4ml mouse monoclonal M5 antibody for the M65 ELISA. Incubation times were 4h and 2h for the M30 and M65 ELISAs, respectively. After washing the plate, 200μl of tetramethylbenzidine (TMP) substrate solution was added to each well followed by stop solution (1M sulfuric acid) after 20 minutes. Absorbance was read in a microplate reader at 450nm. Samples were diluted, as necessary, for the absorbance (450nm) to fall within the range of the standard curve and were done in triplicate.
Determination of IFN α levels
IFNα levels were determined by ELISA (R&D Systems Inc, Minneapolis, MN) utilizing the manufacturer protocol with the extended range standard curve. Briefly, 100μl of each cell culture supernatant and standards were tested in duplicate or triplicate. After one hour incubation and plate washing, 100μl of HRP substrate was added to each well. After incubation for 1h and washing the plate, 100μl TMP substrate solution was added to each well followed by stop solution. Absorbance was read in a microplate reader at 450nm. Samples were diluted, as necessary, for the absorbance (450nm) to fall within the range of the standard curve.
RESULTS
Intron A produces apoptotic cell death in IFN sensitive bladder cells that can be quantitated by M30 ELISA whereas Ad-IFN treatment causes an increase in both M30 and M65 levels
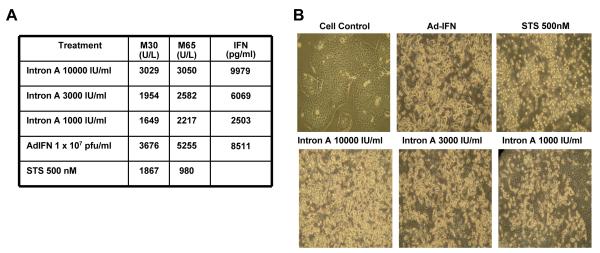
It is known that bladder cancer cells which are sensitive to the IFN protein (Intron A) are killed primarily through a TRAIL-related pathway and in this IFN-induced apoptosis is completely blocked by the caspase 8 selective inhibitor, zIETDfmk (1). Since the RT4 superficial bladder cancer cell line is representative of IFNα sensitive cell lines we wished to document that the M30 ELISA levels would reflect this apoptotic cell death in a concentration dependent manner following IFNα treatment. As shown in Figure 1A this was indeed the case with no significant increase in M65 levels observed over that of M30. However, Ad-IFN treatment resulted in an increase of M65 level over that obtained with the M30 ELISA. The results shown are the average of triplicate readings in which the M30 and m65 levels of the control samples were subtracted.. In addition Ad-β-gal was not cytotoxic to the RT4 cells nor did it produce a significant increase in M30 or M65 levels. These findings indicated that in addition to the apoptotic cell death caused by the IFN protein produced in the cells by the Ad-IFN, there also appeared to be an additional necrotic component of Ad-IFN induced death in these IFNα sensitive cells. Of note the levels of M30 measured in the cells 48h after treatment with 10,000 IU of Intron A was similar to that seen 48h after Ad-IFN exposure. The amount of IFN present in supernatant at this time point was also similar, suggesting that most of the M30 levels observed in the Ad-IFN treated cells were the result of the IFN produced by the Ad-IFN exposure. Staurosporine, known to cause cell death by apoptosis only, also showed an increase in M30 levels with no further increase in M65 levels as predicted if M30 measures only apoptotic cell death (Fig.1A). The large amount of floating dead or dying cells after each treatment is seen by phase-contrast microscopy (Fig. 1B) whereas none was observed after Ad-β-gal treatment (not shown).
Fig.1.
Cytotoxicity and increase in M30 produced by Intron A in IFNα sensitive RT4 bladder cancer cells. A. M30 and M65 levels in the medium of the various treatments shown. No significant increase in M65 was seen compared to M30 levels with any treatment except for Ad-IFN. All values given are those in which the control M30 and M65 levels have been subtracted. Therefore both Intron A and staurosprine produced apoptotic cell death, whereas Ad-IFN caused both apoptotic and necrotic cell death as measured by M30 and M65. B. Phase-contrast micrographs illustrating cell death with numerous floating cells 48h after treatment of RT4 cells with the various concentrations of Intron A as well as Ad-IFN and staurosporine. A concentration dependent increase in cytotoxicity is seen ranging from 1000 to 10,000 IU/ml of Intron A. Magnification 400X. Ad-β-gal was not cytotoxic to the RT4 cells (not shown).
Conditioned medium (CM) from Ad-IFNα infected bladder cancer and normal urothelial cells is cytotoxic to IFNα resistant cancer cells and causes an increase in M30 levels, whereas Ad-IFN produced cytotoxicity in IFN resistant cancer cells is reflected by an increase in M65 levels above that of M30
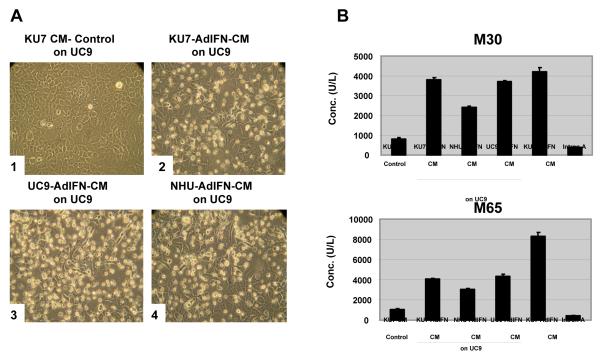
We have recently found that CM from both bladder cancer and normal urothelial cells obtained from Ad-IFN transduced cells are cytotoxic to bladder cancer cells resistant to the IFNα protein which is the result of bystander factor(s) which are produced (4). When the CM from IFN resistant KU7 and UC9 or normal urothelial cells obtained 48h after treatment with Ad-IFN was diluted 1 to 5 with normal medium and placed on IFN resistant UC9 bladder cancer cells considerable cytotoxicity was seen 48h later as shown by phase-microscopy (Fig.2A). Significant increases in M30 could be measured in all CM treatments and the levels obtained were quite similar and reproducible as indicated by the narrow range in values found in the triplicate samples (Fig.2B). This suggested that a major mechanism of cell death produced by the bystander factor(s) involved apoptotic pathways. In addition, no additional increase in M65 was observed in the CM treated cells (Fig.2B), indicating that the bystander-produced cell kill did not include a major necrotic component. In contrast, in the Ad-IFN-CM obtained from the KU7 cells prior to placing in on the UC9 cells was examined, there was an approximate doubling of the M65 levels compared to M30, again indicating that there was an additional necrotic component produced by the direct effect of Ad-IFN on cancer cells. This was likely the direct effect of Ad-IFN transfection and expression in the cancer cells. Ad-βgal in contrast was not cytotoxic to either the UC9 or the KU7 cells nor did it produce M30 or M65 levels above that found in control medium.
Fig.2.
Cytotoxicity produced by CM from Ad-IFN treated bladder cancer and normal urothelial cells on IFNα resistant UC9 bladder cancer cells. A. Numerous floating dead cells are seen in UC9 cells 48h after plating with CM obtained from Ad-IFN treated UC9, KU7 and TERT-NHU cells. Magnification 400X B. An increase in M30 was found for each CM treatment shown in A, indicative of apoptotic cell death caused by the bystander factor(s) present in the CM. No increase in M65 levels were observed above that of M30. As expected, no increase in M30 was found following Intron A treatment, since UC9 cells are resistant to Intron A. Ad-IFN, however, produced increases in both M30 and M65 suggesting that Ad-IFN induces both an apoptotic and necrotic component of cell death.
Ad-IFN treatment of TERT-NHU cells causes no cytotoxicity nor increase in M30 or M65 levels but NHU-AdIFN-CM is consistently cytotoxic to IFN resistant cells reflected by an increase in M30 levels
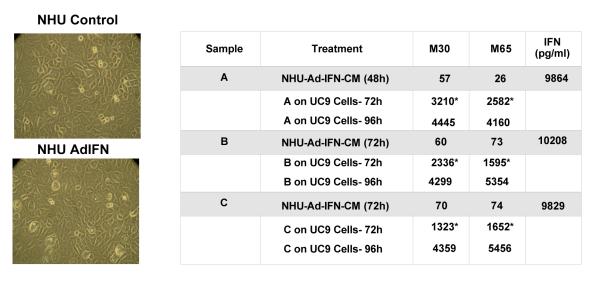
Although a 1 to 5 dilution with normal medium was used when the CM was added to the UC9 target cells as shown in Figure 2B, some of the M30 levels found in the UC9 supernatants examined after the UC9 AdIFN-CM and KU7 AdIFN-CM exposure could have come from the M30 already present in the CM. This should have not been a factor for the TERT-NHU cells, since Ad-IFN produces no cytotoxicity in normal human urothelial cells (Fig.3). To examine this issue in more detail several NHU AdIFN-CMs were examined either 48h or 72h after Ad-IFN treatment. Very low levels of either M30 or M65 were seen in these samples (Fig.3). However, when the NHU AdIFN-CM was added to UC9 for 72h or 96h considerable cytotoxicity and high levels of M30 were observed following Ad-IFN treatment (Fig.3). Little or no increase in M65 over that of M30 was seen (Fig.3).This confirmed that the bystander factor(s) present in the CM produced their cytotoxicity through apoptotic mechanisms that could be measured by M30.
Fig.3.
CM from TERT-NHU cells obtained from Ad-IFN treatment is cytotoxic to UC9 cells and produce increases in M30. Shown in the left lower plate Ad-IFN was not cytotoxic to normal TERT-NHU cells. Magnification 400X. NHU-AdIFN-CM also produced no significant levels either of M30 or M65. However, when various preparations of NHU-AdIFN CM were added to UC9 cells for 72h or 96h, considerable cytotoxicity and increases, primarily in M30 levels were seen. Asterisks indicate results in which the M30 and M65 levels in the control CM results have been subtracted from the levels measured for each of the NHU-Ad-IFN-CM added to UC9 cells for 72 hr. The 96h results are the actual levels recorded with control levels not being subtracted since they were not determined.
Each of the NHU AdIFN-CM samples contained approximately 10,000 pg/ml of IFNα which was the result of Ad-IFN transfection and expression in the NHU cells (Fig.3). Since UC9 cells are IFNα resistant and IFNα produces no significant M30 or M65 levels in these cells (Fig.2), the increase in M30 produced by the NHU AdIFN-CM must reflect the apoptotic cell death caused by the bystander factor(s) rather than the IFN levels present.
DISCUSSION
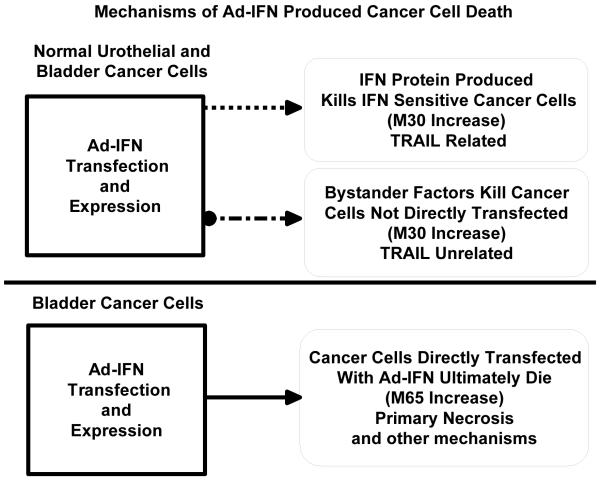
The results provided in this report and elsewhere suggest that several mechanisms are involved in Ad-IFN induced bladder cancer cell death. These are diagrammed in Figure 4. First, both normal and bladder cancer cells produce high and prolonged levels of IFNα following Ad-IFN transfection and expression. If the cancer cells are sensitive to IFNα, such as RT4 cells, they will undergo an apoptotic cell death, largely through a TRAIL-related, caspase-8-dependent mechanism (1). Often in these cases there will be an increase in TRAIL that can be measured (1) and we have shown in this report that this apoptotic cell death can be measured by an increase in M30 levels. If the cancer cells are resistant to IFN, such as the KU7 and UC9 cells, both the normal urothelial and cancer cells still produce bystander factors that can kill the cancer cells by a TRAIL-independent mechanism (3,4). This cell death appears to mostly involve apoptotic pathways since we have found, as reported here, that there is primarily an increase in M30 levels with little or no increase in M65. Finally there is cancer cell death produced directly in cancer cells by Ad-IFN transfection and expression that does not appear to effect normal cells (2,3). We unexpectedly found that this direct cell death apparently is related to necrotic events, since there is an increase primarily in M65 levels after Ad-IFN treatment in IFN resistant cancer cells, once the M30 levels produced by the bystander component is subtracted.
Fig.4.
Diagram outlining proposed mechanisms of Ad-IFN produced cancer cell death. Both normal urothelial and bladder cancer cells produce high and prolonged levels of IFNα following Ad-IFNα exposure which kills the tumor cells through an apoptotic, TRAIL-related mechanism if the cancer cells are sensitive to IFNα. If the cancer cells are resistant to IFNα they still can be killed by a TRAIL-unrelated apoptotic mechanism involving the production of bystander factor(s). Both cause an increase in M30 levels. A direct necrotic cancer cell death is also produced following Ad-IFN transfection and expression which is a reflected in an increase in M65 levels. Other mechanisms of direct cell death may also be involved such as secondary necrosis causing some of the increase in M65 levels.
The M30 and M65 ELISAs are being used in pharmacodynamic studies to look for evidence of apoptotic or necrotic changes in the plasma as potential surrogate markers of tumor cell kill following anticancer treatment (7-9). It was our hypothesis that these ELISAs also could be useful as potential surrogate biomarkers of tumor cell death in the urine following intravesical Ad-IFN instillation. From the results provided in this report we believe this possibility is likely to be correct, since Ad-IFN is not cytotoxic to normal urothelial cells nor produces any increase in M30 or M65 levels. If a given tumor is sensitive to IFNα we would expect that the increase in IFNα concentration in the urine would kill tumor cells resulting in an increase M30 in the urine. If the tumor is resistant to the IFNα, tumor cells could be killed by the bystander factor(s) produced resulting also in an increase in M30 in the urine. It may be possible to differentiate between tumor cell death and the associated M30 increase which is completely bystander related from that in which there is an M30 component caused by the tumor being IFN protein sensitive. In the later case there could be a concomitant increase in TRAIL levels which also can be measured in the urine, whereas there would be none in a IFNα resistant tumor. Finally, an increase in M65 in the urine above that of M30 would likely reflect tumor cell kill involving the direct cytotoxity caused by Ad-IFN transfection and expression of the tumor cells.
Evaluation of M30 and M65 levels in the urine as well as both IFNα and TRAIL levels after intravesical Ad-IFN/Syn3 treatment for superficial bladder cancer is presently in progress. Initial results in the urine from patients after treatment with Ad-IFN/Syn3 have shown not only high levels of IFN but also significant M30 and M65 levels with some patients also having urine containing considerable amount of TRAIL. The final results will be reported once the Phase l trial is completed. However, the information provided in this report provides the basis to enable meaningful interpretation of the M30 and M65 levels found in the urine which can be correlated with urine IFN and TRAIL levels as well as clinical efficacy.
Footnotes
Supported by a GU SPORE in Bladder Cancer (P50 CA91846). MF is supported by T32 NIH grant CA079449.
REFERENCES
- 1.Papageorgiou A, Lashinger L, Millikan R, Benedict WF, Dinney CP, McConkey DJ. Autocrine TRAIL production mediates interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64:8973–8979. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]
- 2.Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, et al. Intravesical Ad-IFNα causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFNα protein. Mol Ther. 2004;10:525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X-Q, Yang Z, Dong L, Papageorgiou A, McConkey D, Benedict WF. Adenoviral-mediated interferon a overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer Gene Ther. 2007;14:241–250. doi: 10.1038/sj.cgt.7701011. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X-Q, Dong L, Chapman E, Benedict WF. Conditioned medium from Ad-IFNα infected bladder cancer and normal urothelial cells is cytotoxic to cancer cells but not normal cells: Further evidence for a strong bystander effect. Cancer Gene Ther. doi: 10.1038/cgt.2008.53. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J, Ranson M, Butt F, Moore D, Dive C. Quatification of M30 and M65 ELISAs as surrogate biomarkers of cell death: long term antigen stability in cancer patient plasma. Cancer Chemother Pharmacol. 2007;60:921–924. doi: 10.1007/s00280-007-0437-4. [DOI] [PubMed] [Google Scholar]
- 6.Linder S. Cytokeratin markers come of age. Tumor Biol. 2007;28:189–195. doi: 10.1159/000107582. [DOI] [PubMed] [Google Scholar]
- 7.Kramer G, Erdal H, Mertens MM, Nap M, Mauermann J, Steiner G, et al. Differentiation between cell death modes using measurement of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751–1756. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J, Ranson M, LaCasse E, Ganganagari JR, St-Jean M, Jayson G, et al. Method validation and preliminary quantification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Med. 2006;95:42–48. doi: 10.1038/sj.bjc.6603220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, Knowles MA. Expression of hTERT immortalizes normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene. 2006;25:5037–5045. doi: 10.1038/sj.onc.1209513. [DOI] [PubMed] [Google Scholar]