Abstract
Atrial fibrillation is an important comorbidity with substantial therapeutic implications in dialysis patients but its prevalence varies in different studies. We used a database that includes patients in the United States on hemodialysis who were eligible for government assistance with prescription drugs. We then used ICD-9 codes from billing claims in this database to identify patients with chronic atrial fibrillation. Multivariable logistic regression was used to determine adjusted prevalence odds ratios for associated factors. Of 63,884 individuals, the prevalence of chronic atrial fibrillation was 7%. The factors of age over 60 years, male, Caucasian, body mass index over 25 kg/m2, coronary artery disease, and heart failure were all significantly associated with chronic atrial fibrillation. Prevalence rates, particularly in younger patients, were far higher than those reported in an age group–matched nondialysis population. Thus, given its clinical impact, future efforts are needed to examine risk factors for adverse outcomes in chronic atrial fibrillation, and to identify appropriate management strategies for this disorder, as well as opportunities for quality improvement in this vulnerable population.
Keywords: atrial fibrillation, dialysis, end-stage renal disease, prevalence
Atrial fibrillation (AF) is common in end-stage renal disease (ESRD) patients on maintenance dialysis,1–9 is independently associated with mortality in both general10 and dialysis8,11 populations, and has important therapeutic implications. A full understanding of chronic AF prevalence, factors associated with its prevalence, and the outcomes of ESRD patients with chronic AF could be used to inform practitioners about the patterns of current health-care delivery and opportunities for improvement. Although recent studies examining the epidemiology of chronic AF in cohorts drawn from a large dialysis-provider organization,12 from the Dialysis Outcomes and Practice Patterns Study (DOPPS),8 and in United States Renal Data System (USRDS) data13 signal renewed interest in this area, estimates of chronic AF prevalence remain quite variable, ranging from 6%1 to nearly 27.0%.7,8 The wide variability of these estimates stems, in part, from differences in individual study samples, study design (for example, prospective cohort vs. retrospective cohort vs. cross-sectional studies), classification of AF (for example, chronic vs. transient or perioperative), and diagnostic strategies (for example, self-reports vs. targeted identification in longitudinal cohorts vs. retrospective records review). Given this variability in the prevalence estimates for chronic AF, additional work to rigorously characterize the prevalence of this disorder and its associated factors is needed to inform health-care decision making, especially in vulnerable populations, such as those receiving government financial assistance.
To study patients with chronic AF, we constructed a novel database. This database links US Medicare data, which contain federal billing claims for inpatient and outpatient medical services permitting inferences about medical conditions, with Medicaid data for state-supported individuals, which contain prescription drug records. We then used an algorithm, originally developed for identifying chronic AF in the general population,14,15 to determine the prevalence of this disorder in dialysis patients. We elected to study patients with both Medicare and Medicaid (‘dually-eligibles’) because they represent a particularly vulnerable and resource-intensive subgroup of patients, and they have readily available prescription medication records (a required component of our identification algorithm) by virtue of their Medicaid eligibility (http://www.npcnow.org/Public/Research___Publications/Publications/pub_rel_research/pub_medicaid/Pharmaceutical_Benefits_Under_State_Medical_Assistance_Programs_2003.aspx). Our aims were to provide a comprehensive examination of the prevalence of, and factors associated with, chronic AF, an important disorder with major clinical implications.
RESULTS
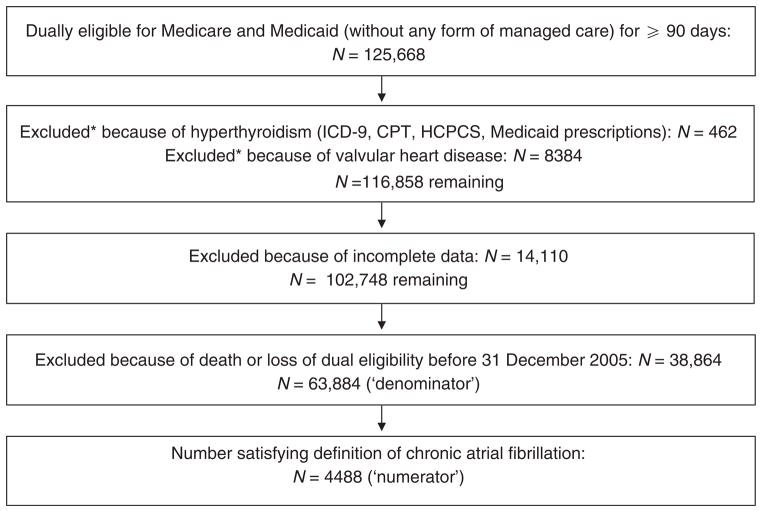
A total of 430,227 individuals were receiving chronic dialysis in 2004 and 2005. Figure 1 demonstrates the number of individuals retained at each step of cohort construction. A total of 125,668 individuals were dually eligible for at least 90 days. A small number of individuals (n =462) were excluded because of hyperthyroidism, whereas a larger number of them (n =8384) were eliminated because of valvular heart disease, leaving 116,858 individuals. Elimination of individuals with incomplete data left 102,748 individuals; the vast majority of attrition occurred because information on body mass index (BMI) and/or hemoglobin level was missing. A total of 63,884 individuals were alive on 31 December 2005, of whom 4488 satisfied the definition of chronic AF (7.0%).
Figure 1. Exclusion flowchart demonstrating the creation of the study cohort.
*Some individuals were excluded because of both hyperthyroidism and valvular heart disease. CPT, Common Procedural Terminology; HCPCS, Healthcare Common Procedural Coding System; ICD-9, International Classification of Diseases–Ninth Revision.
Mean age of the overall cohort was 60.0 ± 15.1 years. Women comprised 53.0%, of whom 31.4% were Caucasian, and 45.8% were African American. Well over half (60.8%) of the patients with chronic AF had diabetes. Bivariate analyses of the differences in characteristics between patients with and without chronic AF are shown in Table 1. As noted, 7.0% (95% confidence interval (CI) 6.8–7.2%) had chronic AF. Patients with chronic AF were significantly (P < 0.01) more likely to be older (70.0 vs. 59.3 years), female, Caucasian, have higher BMI values, be nonsmokers, be unemployed, be unable to ambulate and transfer, and have diabetes mellitus, congestive heart failure, coronary artery disease (CAD), peripheral vascular disease, and a history of a cerebrovascular accident; hypertension (HTN) was less common in patients with chronic AF. Patients with the disorder were also more likely to be on in-center hemodialysis and to have a hemoglobin level of < 11.0 g/dl.
Table 1.
Characteristics of dialysis patients with and without chronic AF
| Characteristic | Total (n=63,884) | AF (n=4488) | Non-AF (n=59,396) | P-value |
|---|---|---|---|---|
| Age, years | 60.0 ± 15.1 | 70.0 ± 11.8 | 59.3 ± 15.0 | <0.0001 |
| Female sex, n (%) | 33,827 (53.0) | 2519 (56.1) | 31,308 (52.7) | <0.0001 |
| Race/ethnicity, n (%) | <0.0001 | |||
| African American | 29,229 (45.8) | 1445 (32.2) | 27,784 (46.8) | |
| Caucasian | 20,027 (31.4) | 2191 (48.8) | 17,836 (30.0) | |
| Hispanic | 10,532 (16.5) | 551 (12.3) | 9981 (16.8) | |
| Other | 4096 (6.4) | 301 (6.7) | 3795 (6.4) | |
| BMI category, n (%) | <0.0001 | |||
| <20 kg/m2 | 5618 (8.8) | 338 (7.5) | 5280 (8.9) | |
| 20–24.9 kg/m2 | 17,988 (28.2) | 1079 (24.0) | 16,909 (28.5) | |
| 25–29.9 kg/m2 | 17,310 (27.1) | 1238 (27.6) | 16,072 (27.1) | |
| >30 kg/m2 | 22,968 (36.0) | 1833 (40.8) | 21,135 (35.6) | |
| Smoker, n (%) | 4264 (6.7) | 213 (4.8) | 4051 (6.8) | <0.0001 |
| Substance abuser, n (%) | 2193 (3.4) | 75 (1.7) | 2118 (3.6) | <0.0001 |
| Unemployed, n (%) | 59,861 (93.7) | 4333 (96.6) | 55,528 (93.5) | <0.0001 |
| Inability to ambulate, n (%) | 2388 (3.7) | 305 (6.8) | 2083 (3.5) | <0.0001 |
| Inability to transfer, n (%) | 806 (1.3) | 117 (2.6) | 689 (1.2) | <0.0001 |
| Primary cause of ESRD, n (%) | <0.0001 | |||
| Diabetes | 31,230 (48.9) | 2239 (49.9) | 28,991 (48.8) | |
| Hypertension | 17,480 (27.4) | 1252 (27.9) | 16,228 (27.3) | |
| Glomerulonephritis | 6315 (9.9) | 316 (7.0) | 5999 (10.1) | |
| Other | 8859 (13.9) | 681 (15.2) | 8178 (13.8) | |
| Comorbidities, n (%) | ||||
| Hypertension | 54,886 (85.9) | 3797 (84.6) | 51,089 (86.0) | 0.009 |
| Diabetes | 37,001 (57.9) | 2728 (60.8) | 34,273 (57.7) | <0.0001 |
| CHF | 17,619 (27.6) | 1926 (42.9) | 15,693 (26.4) | <0.0001 |
| CAD | 11,863 (18.6) | 1400 (31.2) | 10,463 (17.6) | <0.0001 |
| PVD | 6996 (11.0) | 733 (16.3) | 6263 (10.5) | <0.0001 |
| CVA | 5257 (8.2) | 518 (11.5) | 4739 (8.0) | <0.0001 |
| Self-care dialysis, n (%) | 3401 (5.3) | 171 (3.8) | 3230 (5.4) | <0.0001 |
| Hb <11.0 g/dl, n (%) | 48,997 (76.7) | 3342 (74.5) | 45,655 (76.9) | 0.0002 |
Abbreviations: AF, chronic atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; ESRD, end-stage renal disease; Hb, hemoglobin; PVD, peripheral arterial disease.
The P-values were from Pearson’s χ2 test for categorical measures and the two-sample t-test for continuous measures.
Table 2 illustrates the result of multivariable modeling. Age > 60 years, male sex, Caucasian race, BMI ≥25 kg/m2, lack of smoking, inability to ambulate, histories of CAD congestive heart failure, and HTN as the cause of ESRD, compared with those with diabetes mellitus as a cause of ESRD and HTN as a comorbidity, were significantly (P < 0.01) associated with chronic AF. HTN was inversely associated with chronic AF; specifically, the adjusted prevalence odds ratio (APOR) of 0.79 indicates that, among those individuals in whom HTN was not the primary cause of ESRD, there was a lesser prevalence of chronic AF relative to those who did not have HTN as a comorbidity on the CMS 2728 form. The use of self-care dialysis demonstrated borderline significance (P =0.011), whereas diabetes and a hemoglobin level of < 11 g/dl were not associated with chronic AF. No lack of fit was detected for this model (P > 0.05).
Table 2.
APOR estimates for dually eligible (Medicare and Medicaid) dialysis patients with chronic atrial fibrillation
| Characteristic | APOR | 95% CIs | P-value |
|---|---|---|---|
| Age >60 years | 3.54 | 3.27–3.84 | <0.0001 |
| Male sex | 1.12 | 1.05–1.20 | 0.0005 |
| Race/ethnicity | |||
| Caucasian | — | — | — |
| African American | 0.51 | 0.47–0.55 | <0.0001 |
| Hispanic | 0.51 | 0.46–0.56 | <0.0001 |
| Other | 0.71 | 0.62–0.81 | <0.0001 |
| BMI category | |||
| <20 kg/m2 | 1.01 | 0.89–1.15 | 0.87 |
| 20–24.9 kg/m2 | — | — | — |
| 25–29.9 kg/m2 | 1.20 | 1.10–1.31 | <0.0001 |
| >30 kg/m2 | 1.47 | 1.35–1.59 | <0.0001 |
| Smoker | 0.80 | 0.69–0.93 | 0.003 |
| Substance abuser | 0.83 | 0.65–1.06 | 0.13 |
| Employed | 0.87 | 0.74–1.03 | 0.12 |
| Inability to ambulate | 1.28 | 1.10–1.50 | 0.002 |
| Inability to transfer | 1.16 | 0.91–1.48 | 0.25 |
| Comorbidities | |||
| Hypertensiona | 0.79 | 0.72–0.87 | <0.0001 |
| Diabetesa | 0.90 | 0.80–1.00 | 0.051 |
| CAD | 1.29 | 1.20–1.39 | <0.0001 |
| CHF | 1.56 | 1.45–1.67 | <0.0001 |
| CVA | 1.11 | 1.00–1.22 | 0.055 |
| PVD | 1.05 | 0.96–1.15 | 0.29 |
| Cause of ESRD | |||
| HTN vs. DMb | 1.26 | 1.13–1.41 | <0.0001 |
| HTN vs. GNc | 1.11 | 0.98–1.270 | 0.12 |
| HTN vs. Otherc | 1.10 | 0.99–1.22 | 0.089 |
| DM vs. GNd | 0.88 | 0.76–1.03 | 0.13 |
| DM vs. Otherd | 0.87 | 0.77–0.99 | 0.031 |
| Other vs. GNe | 1.02 | 0.88–1.17 | 0.82 |
| Self-care dialysis | 0.81 | 0.69–0.95 | 0.011 |
| Hb <11.0 g/dl | 0.98 | 0.92–1.06 | 0.67 |
Abbreviations: APOR, adjusted prevalence odds ratio; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CVA, cerebrovascular accident; DM, diabetes mellitus; ESRD, end-stage renal disease; GN, glomerulonephritis; Hb, hemoglobin; HTN, hypertension; PVD, peripheral arterial disease.
As patients were classified as having HTN, for analytic purposes, whether HTN was a cause of ESRD or was listed as a comorbidity on the CMS 2728 form, the APOR represents the association of HTN with chronic atrial fibrillation only in individuals who did not have HTN as the cause of ESRD. The APOR for DM is conceptually identical.
Among individuals with both HTN and DM as comorbidities.
Among individuals with HTN.
Among individuals with DM.
Among individuals irrespective of HTN or DM status.
To facilitate comparison with data in the general population, we calculated raw percentages of chronic AF by age, stratified by decade and sex. Table 3 displays these percentages. As expected, our rates were consistently higher than those of the general population across age strata.15 Absolute rates were roughly twofold higher in the oldest individuals (> 75 years) but > 10-fold in younger individuals (age < 55 years). In young individuals aged 40–44.9 years (not specifically shown in Table 3), 1.4% of women and 2.2% of men had chronic AF, whereas approximately one in six individuals of > 85 years had it. These findings demonstrate that chronic AF both occurs at a younger age in the dialysis patients and has sustained high prevalence rates in the elderly.
Table 3.
Prevalence of chronic atrial fibrillation in dialysis patients compared with an ambulatory patient population, by age decade and sex
| Age, years | Females
|
Males
|
||
|---|---|---|---|---|
| Dialysis % | Ambulatory %a | Dialysis % | Ambulatory %a | |
| <55 | 1.9 (186/9967) | 0.1 | 2.5 (339/13,528) | 0.2 |
| 55 to 60 | 4.7 (181/3811) | 0.4 | 5.4 (216/3990) | 0.9 |
| 60 to 65 | 5.7 (214/3764) | 1.0 | 7.1 (221/3094) | 1.7 |
| 65 to 70 | 8.0 (358/4462) | 1.7 | 9.2 (292/3190) | 3.0 |
| 70 to 75 | 11.6 (500/4304) | 3.4 | 12.2 (305/2508) | 5.0 |
| 75 to 80 | 13.0 (463/3556) | 5.0 | 14.8 (287/1935) | 7.3 |
| 80 to 85 | 15.1 (373/2470) | 7.2 | 16.6 (195/1178) | 10.3 |
| >85 | 16.3 (244/1493) | 9.1 | 18.0 (114/634) | 11.1 |
Estimates for ambulatory patients adapted from Go et al.15
To determine the robustness of our findings, we performed several sensitivity analyses. Using both the more liberal (≥2 claims, without consideration of an episode-of-care window) and the more conservative (≥3 or claims without consideration of the episode-of-care window) approaches, the estimated percentage of individuals with chronic AF was 8.0% (95% CI 7.8–8.2%) and 5.5% (95% CI 5.3–5.7%), respectively. Logistic regression modeling to determine APORs for covariates using the conservative approach produced results very similar to those of our primary analysis, with the only difference being that smoking and inability to ambulate were no longer significant, although the magnitude of the effects were nearly identical; also, male sex became marginally insignificant (P =0.018), although its magnitude of the APOR (1.09) was nearly identical to that obtained earlier (1.12).
To determine whether our analysis in dually eligible patients could be extrapolated to the larger body of chronic dialysis patients, we ran a comparable series of analyses, using the primary approach of ≥2 claims plus the episode-of-care window restriction, in the entire group of Medicare dialysis patients (that is, irrespective of Medicaid eligibility). In this cohort of 173,606 individuals, the overall raw percentage of individuals with chronic AF was higher, at 9.6%, compared with the 7.0% of the comparable approach in dually eligible patients. However, mean age of the patients in this group was substantially higher than that in the dually eligible group (63.6 vs. 59.4 years, respectively). Modeling in this group, shown in Supplementary Table S1 online, yielded generally similar results as before; all previously identified associations remained intact, but the use of in-center hemodialysis now became highly statistically significant, as did employment. The APORs for most factors, however, were very similar to those for the dually eligible patients (differing only at the hundredths place), likely indicating the effect of the increased power of the far larger Medicare cohort. Lack of HTN, as a comorbidity, was once again associated with chronic AF.
DISCUSSION
In this study, we adapted a validated billing claims-based algorithm for the identification of patients with chronic AF in dialysis patients who are dually eligible for both Medicare and Medicaid. We found that 7.0% of these individuals had chronic AF. Our study compliments and expands upon previous reports in several ways. First, we provide a precise estimate of chronic AF prevalence in the dually eligible dialysis population. Compared with other types of patients, those with Medicare and Medicaid are disproportionately likely to be women, African American, smokers, substance abusers, and have functional physical limitations,16 and are associated with disproportionate health-care costs, making them a particularly important vulnerable group of patients to study. Second, we demonstrate an approach through which claims data can be used for identification of chronic AF in dialysis patients. Finally, our large sample size permits fairly precise quantification of the associations between chronic AF prevalence and a variety of demographic, anthropometric, risk behavior, functional status, comorbidity, dialysis modality, and laboratory-value factors as recorded upon dialysis initiation, providing direction for future study.
A validated Medicare claims-based algorithm for identifying chronic AF has been published in several widely disseminated reports.14,15,17 We amended this approach because manual inspection of the claim patterns in several hundred patient records made it likely that many ‘outpatient’ AF claims were likely to be associated with a proximate AF-related hospital admission and were therefore potentially transient. Our episode-of-care window paradigm can minimize misclassification of acute AF as being chronic and improve the accuracy of future efforts to assess and improve the quality of AF treatment in ESRD patients. The robustness of our findings is supported by several sensitivity analyses, using more liberal and conservative approaches, which had minimal impact on our prevalence estimates. Importantly, regardless of the approach used or the sample studied, modeling of the associations between covariates and chronic AF led to consistent APORs.
Our raw prevalence estimates appear to be somewhat lower than some,6–8 but not all,12 previous reports. In terms of raw percentages, the DOPPS investigators recently reported a prevalence rate of 11.3–24.7% in Western countries, with a US rate of 12.5%.8 However, our empiric inspection of AF claims patterns in several hundred patients suggested that some previously reported estimates may be implausibly high. Recent work from a small, but in-depth, study of 256 patients revealed that whereas 7.4% of patients had permanent AF, 12.1% had any type of AF,11 suggesting that a substantial proportion of individuals labeled as having AF are likely to have transient AF or AF due to secondary causes. Thus, high AF rates reported in other studies may be because individuals with secondary causes of AF or transient AF are being captured, and perhaps misclassified, by their respective identification strategies. Additional evidence for lower AF rates comes from a recent report by Chan et al.,12 who concluded that AF rates were ‘only’ 4.5% in incident dialysis patients. It seems likely that rates would become higher in dialysis populations consisting of more ‘prevalent’ individuals, as increasing age, a concomitant growing comorbidity burden, and increased exposure to the hemodialysis procedure itself (a physiological ‘stress test,’ particularly in the United States where rapid ultrafiltration is commonplace) might be expected to result in increased rates of chronic AF. A recent report by Winkelmayer et al.13 found chronic AF rates in the general Medicare dialysis population to be 10.7% in 2006. The rate in their large and rigorous study may be somewhat higher than that of ours because those investigators used a somewhat more liberal definition than did we to establish chronic AF (for example, by requiring only 14 days between AF claims).
Our large study enables more precise estimates of prevalence and risk factors. As such, previously reported findings of associations between various patient factors upon dialysis initiation and chronic AF can be well quantified. As expected, we echoed the finding of the association of age with AF reported in the DOPPS database,8 the Dialysis Morbidity and Mortality (DMMS) Wave II study,18 the recent USRDS study by Winkelmayer et al.,13 and others.5,7,11 Caucasian race has also been found to be associated with AF,8,13,18 as has increasing BMI.8 Notably, the role of sex in dialysis patients with AF as a whole remains unclear; although some investigators report sex to be unassociated with either incident or prevalent AF,5,7,8 and others report that female sex is the risk factor,11 our finding of an association of male sex with chronic AF is consistent with findings in the general population15 and in another recent large study of dialysis patients.13 The counterintuitive finding that smoking was inversely related to chronic AF prevalence is probably because the patients with the greatest comorbidity burden are generally counseled most heavily to cease, at least in the setting of advanced chronic kidney disease. As anticipated, CAD and congestive heart failure emerged as important comorbidites associated with chronic AF.8,11,13
One noteworthy finding was an inverse association, after adjustment for other factors, between HTN and chronic AF in our study. Among individuals without HTN as their primary cause of ESRD, chronic AF was less common in individuals with HTN as a comorbidity (defined as having HTN designated as a CMS 2728 comorbidity) than in those without. However, specific examination of individuals with HTN as a CMS 2728 comorbidity demonstrated that, among those with HTN as the cause of ESRD, the APORs for HTN as the cause of ESRD compared with those with diabetes mellitus as the cause of ESRD was significantly > 1.0 (APOR ≈ 1.3); a similar, although nonsignificant, trend for both glomerulonephritis and other causes of ESRD was observed (both APORs ≈ 1.1). This overall finding is consistent with clear demonstrations that long-standing HTN (such as that severe enough to cause ESRD) is a risk factor for AF in the general population.19–24 However, studies of prevalent dialysis patients demonstrate that they may be characterized by a distinct physiology, namely the controversially termed phenomenon of ‘reverse epidemiology,’25,26 and this may explain how HTN as a CMS 2728 comorbidity could be associated with a decreased likelihood of chronic AF among people who did not have HTN as their cause of renal failure (that is, among people who apparently did not have HTN severe enough to cause ESRD). Evidence from the DOPPS database appears to support our results: although a formal diagnosis of HTN was not associated with AF, low predialysis systolic blood pressure was,8 lending plausibility to the hypothesis that when patients with advanced kidney disease or ESRD manifest AF, many of them are likely to be experiencing decreased cardiac output and concomitant hypotension. As AF may contribute to hypotension in dialysis patients, this might be an explanation for our findings.
Our study should be interpreted in the context of several potential limitations. First, we did not have access to medical charts and relied upon extensive administrative data. However, we adapted a well-established validated algorithm to leverage these data to minimize the possibility that secondary, transient, valvular, or postoperative causes of AF would be misclassified as truly chronic AF. Second, we used the CMS 2728 form to identify comorbidities, as is widely done.27–33 Although this form has several strengths, a more rigorous, but far more complex, approach would be to supplement these diagnoses with a claims-based approach to identify comorbidities. Such an approach exists,34,35 and should be explored in future investigations. It would, however, require a minimum survival of 9 months and would introduce other biases into the analyses.36 Third, our primary analysis was confined to dually eligible individuals. As individuals with Medicaid are likely to be the most financially needy and resource intense, and to have more comorbidities at a younger age than the non-dually eligible population,16 our results can only be generalized with much caution. For example, in patient populations with fewer African-origin individuals, the overall rates are likely to be higher. However, we did perform an additional analysis in the general dialysis population with Medicare, and found consistent APORs for the various covariates. Finally, given the retrospective nature of this study with its point-prevalence approach, we cannot infer causality; we cannot, for example, declare that a marker of functional status (for example, inability to ambulate) ‘predicts’ the development of chronic AF. Rather, the purpose of this study was to elucidate broad associations worthy of future detailed study.
In conclusion, we used a billing claims-based approach to determine the prevalence of chronic AF, an important comorbidity with significant clinical implications, in a vulnerable and resource-intense group of dialysis patients, namely those dually eligible for Medicare and Medicaid. As expected, the prevalence rate is far higher than that of the nondialysis population, a finding that is particularly striking in younger individuals. Male sex, Caucasian race, increasing BMI, inability to ambulate, and the presence of CAD and heart failure are also associated with the disorder. Further investigation of the role these factors have in chronic AF could provide an opportunity to provide better care in this resource-intense group of dialysis patients, and in the dialysis population as a whole.
MATERIALS AND METHODS
Study design
A retrospective cohort analysis of patients undergoing maintenance dialysis during the 2-year period of 1 January 2004 to 31 December 2005 was used to identify people with chronic AF, as described below. The intention was to study individuals with persistent or recurrent (and therefore chronic) AF, rather than individuals with transient (typically postoperative) AF or AF due to structural heart disease. We identified chronic AF in patients who were observable for at least 3 months during the 2-year window, after having initially survived their first 90 days on dialysis, and who were alive with dual eligibility on 31 December 2005 to estimate the point prevalence of chronic AF.
Data sources for analysis
Our data were derived from two primary sources. The first was the USRDS, a national data system that collects and analyzes data on virtually all patients undergoing chronic dialysis in the United States. The USRDS incorporates data on inpatient and outpatient medical claims paid by Medicare, a federally funded health-care program that pays health-care costs (excluding prescription drugs until 2006) for the vast majority of dialysis patients. Medicare claims, which are commonly used in secondary data analyses in the United States,17,37–40 contain International Classification of Diseases–Ninth Revision (ICD-9) codes keyed to specific dates of service. As such, when a provider (such as a physician or hospital) submits a billing claim to Medicare, the presence of a medical condition in a specific patient at a specific time can be inferred.
The other source of data was Medicaid prescription drug billing claims. Medicaid is a program in the United States that is designed to assist the most financially needy individuals with health-care costs, particularly prescription drugs. Medicaid data from all 50 states were used to identify prescriptions at the individual patient level. As our definition of chronic AF required medical claims data (from Medicare), as well as information on prescription medications (from Medicaid), these sources were linked through a multistep process shown in detail in Supplementary Figure S1 online. This process permitted us to identify dually eligible (Medicare & Medicaid) dialysis patients in 2004 and 2005.
We acquired patient demographic characteristics, comorbid conditions, laboratory values before initiation of chronic dialysis, date of dialysis initiation, and dialysis modality from the USRDS from the Medical Evidence Form (the ‘CMS 2728’ form). This detailed form is completed by providers for all patients when they initiate dialysis, and is subsequently incorporated in the USRDS database. All covariates used for the analysis were therefore established before a determination of the chronic AF was undertaken from the claims data.
Study cohort and rationale for analytical approach
We identified unique individuals above the age of 20 years who survived > 90 initial days on dialysis, who were Medicare eligible for at least 90 days, who were simultaneously enrolled in Medicare and Medicaid programs for at least 90 days during the 2-year observation window, and who were alive (and still dually eligible) on 31 December 2005. We excluded individuals who initiated dialysis before 1 January 1980, who were enrolled in the Veterans Administration health-care system or any form of managed care (and thus for whom we would not have complete records billing claims or prescription records), and who received a transplant before 1 January 2004. We censored patients if they lost eligibility for either Medicare or Medicaid.
Determination of chronic AF
The ICD-9 code 427.31 was used to identify AF claims.14 To determine the presence of nontransient, nonvalvular AF, we used an algorithm adapted from Go et al.14,15,17 The cohort construction strategy, relevant exclusions, and impact on sample size are depicted in Figure 1. We began by eliminating individuals who had hyper-thyroidism or thyrotoxicosis based on the presence of relevant ICD-9 and/or CPT (Common Procedural Technology) and/or HCPCS (Healthcare Common Procedure Coding System) codes, or by a prescription at any time for methimazole or propothiouracil.14,15 Drug exclusions were defined by matching name and therapeutic class information from the Medicaid prescription drug files with National Drug Codes from Multum Lexicon (Cerner Corporation, Kansas City, KS). Next, we eliminated patients with evidence of valvular heart disease (using ICD-9 codes). We then eliminated individuals with incomplete data on the CMS 2728 dialysis intake form. Finally, to minimize potential misclassification from perioperative sources of AF (for example, coronary artery bypass surgery), claims (rather than individuals) were eliminated unless there was a preexisting (> 30 days) AF claim. This resulted in the elimination of individuals in whom AF claims were only proximally related to cardiac surgery.14,15 A specific accounting of this exclusionary strategy is listed in detail in Supplementary Table S2 online.
To classify individuals as having chronic AF, we initially required a total of two (or more) AF claims, separated by ≥30 days, of which no more than one was an inpatient claim.14,15 However, manual inspection of the claims patterns of > 200 individuals with AF claims revealed that a large number of outpatient AF claims appeared immediately before and/or soon after an extended hospitalization that contained an AF claim. To help rigorously establish the presence of truly chronic AF, we formulated criteria for an ‘episode-of-care’ window. Because we wanted to guard against overattribution (that is, giving inappropriate credit to multiple AF claims when they truly reflected only a single episode of care), we expunged all outpatient AF claims within 7 days of a subsequent AF claim-containing admission, thus retaining only that inpatient claim. Similarly, as outpatient follow-up care for AF could be closely tied to a recent hospital admission for AF, we expunged all outpatient AF claims within 30 days after an AF claim-containing admission, retaining only the original inpatient claim. By establishing the 7-day pre- and 30-day post-admission windows, additional AF claims likely to be associated with a single episode of care were eliminated, whereas a single claim associated with the hospitalization was retained.
We performed several sensitivity analyses. First, we used a more liberal approach that did not include the episode-of-care window14,15 to capture individuals with chronic AF who might have been missed with our primary approach. Second, because dialysis patients have increased contact with the health-care system, and therefore have more opportunities to acquire AF claims than do other nondialysis-requiring ambulatory patients, we amended the original algorithm used by Go et al.14,15 by requiring a third AF claim (no more than one of which could be an inpatient claim) in order to determine how this more conservative approach would affect our results. Finally, to compare our results with the larger dialysis population, we performed one additional analysis in which the full Medicare population (irrespective of Medicaid eligibility, and therefore more representative of the US dialysis population as a whole) was investigated, using the two-claim approach with the episode-of-care window invoked (that is, the identical approach used in the primary analysis for the dually eligible patients). It is noteworthy that as only a subset of individuals with Medicare have Medicaid, and because we relied on Medicaid prescription records to eliminate people with presumptive hyperthyroidism (an important cause of chronic AF), the definition of chronic AF in the broader Medicare population could not use information from prescription records.
Independent variables
A variety of covariates, as recorded on the CMS 2728 dialysis intake form, were considered for analysis. The relationship of each covariate to include with the model with the outcome was considered a priori. Dichotomous variables were classified as being present or absent at the time of dialysis initiation. Demographic variables were age, sex, and race by ethnicity (four mutually exclusive groups consisting of non-Hispanic Caucasian, non-Hispanic African American, Hispanic, and Others). BMI was classified into four categories: < 20, 20–24.99, 25–29.99, and ≥30 kg/m2. Risk behavior factors examined were smoking and substance abuse (alcohol or illicit drugs), and functional status markers were employment, inability to ambulate, and inability to transfer. Major comorbidities were considered to be diabetes (types 1 and 2 combined), HTN, congestive heart failure, CAD, cerebrovascular disease, peripheral vascular disease, and cardiac dysrhythmia. As the CMS 2728 form is structured such that diseases such as diabetes or HTN may be considered as both a cause of ESRD and/or a ‘freestanding’ comorbidity, for the purposes of the present analysis, diabetes and HTN were considered a comorbidity if they were listed as either the cause of ESRD or as a freestanding comorbidity on the CMS 2728 form.27 This approach therefore required a series of linear contrasts of the model parameters for statistical inference (see below). Modality upon initiation of dialysis was categorized as in-center hemodialysis or self-care dialysis (home hemodialysis or peritoneal dialysis). To examine the association of anemia, which has been identified as an important AF predictor in the nondialysis population, we included baseline hemoglobin at baseline, which was dichotomized at 11 g/dl. Serum albumin was not analyzed, as ~ 20% of individuals did not have this value recorded.
Statistical analyses
We generated descriptive statistics (means for continuous variables and percentages for categorical variables) for the patients with and without chronic AF. To explore the differences between two groups (that is, those with and without chronic AF) through bivariate analyses, we performed Pearson’s χ2 tests for categorical measures and the two-sample t-test for continuous measures. Raw percentages of individuals who ever satisfied the definition of chronic AF (irrespective of length of follow-up time) were generated for each of the approaches, and 95% Wald CIs were generated. The number of individuals with chronic AF on 31 December 2005 was ascertained, and multivariable logistic regression was used to estimate adjusted prevalence odds ratios for factors associated with chronic AF. Age was dichotomized at 60 years to improve fit of the model. APORs for chronic AF were generated for each covariate. Lack of fit for this model was evaluated using the Hosmer–Lemeshow goodness-of-fit test.
We used a P-value of < 0.01 to be the threshold for statistical significance. This approach allowed us to reduce the likelihood of a type 1 error with (presumably) minimal impact on the power to detect significant finding given the large sample size. All statistical analyses were performed with SAS 9.2 (Cary, NC).
Compliance and protection of human research participants
The research protocol was approved by the institutional review board at the University of Kansas Medical Center (KUMC), and the project was undertaken according to the Declaration of Helsinki Principles. Data Use Agreements (DUA) between KUMC and the USRDS and CMS permitted the data linking across the USRDS, Medicare, and Medicaid files. In accordance with our DUA, social security numbers and other identifying information were removed from the linked files provided by CMS and the USRDS.
Acknowledgments
We thank Connie Wang for technical assistance with manuscript preparation. Funding for this study was provided by the NIH (NIDDK) grants R01 DK080111-02 (to TIS) and K23 DK085378-01 (to JBW), a National Kidney Foundation Young Investigator Award (to JBW), and a Sandra A. Daugherty Foundation grant (to JBW).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Disclaimer
The data reported here have been supplied by the United States Renal Data System (DUA 2007–10 and 2009–19) and the Centers for Medicare and Medicaid Services (DUA 19707). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
SUPPLEMENTARY MATERIAL
Table S1. Adjusted prevalence odds ratio (APOR) estimates for dialysis for Medicare patients (irrespective of Medicaid eligibility) with chronic atrial fibrillation.
Table S2. Sources of exclusion of individuals with potential secondary sources of atrial fibrillation.
Figure S1. Conceptual model of the matching strategy used to construct the study cohort.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
References
- 1.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. USRDS. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2005. 2005. [Google Scholar]
- 3.Abe S, Yoshizawa M, Nakanishi N, et al. Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J. 1996;131:1137–1144. doi: 10.1016/s0002-8703(96)90088-5. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez E, Sanchez-Perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J. 2000;140:886–890. doi: 10.1067/mhj.2000.111111. [DOI] [PubMed] [Google Scholar]
- 5.Atar I, Konas D, Acikel S, et al. Frequency of atrial fibrillation and factors related to its development in dialysis patients. Int J Cardiol. 2006;106:47–51. doi: 10.1016/j.ijcard.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Fabbian F, Catalano C, Lambertini D, et al. Clinical characteristics associated to atrial fibrillation in chronic hemodialysis patients. Clin Nephrol. 2000;54:234–239. [PubMed] [Google Scholar]
- 7.Genovesi S, Pogliani D, Faini A, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. doi: 10.1053/j.ajkd.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 8.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 9.Korantzopoulos P, Kokkoris S, Liu T, et al. Atrial fibrillation in end-stage renal disease. Pacing Clin Electrophysiol. 2007;30:1391–1397. doi: 10.1111/j.1540-8159.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez E, Sanchez-Perales C, Garcia-Garcia F, et al. Atrial fibrillation in incident dialysis patients. Kidney Int. 2009;76:324–330. doi: 10.1038/ki.2009.185. [DOI] [PubMed] [Google Scholar]
- 12.Chan KE, Lazarus JM, Thadhani R, et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkelmayer WC, Patrick AR, Liu J, et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 16.Wetmore JB, Rigler SK, Mahnken JD, et al. Considering health insurance: how do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant. 2010;25:198–205. doi: 10.1093/ndt/gfp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 18.Abbott KC, Trespalacios FC, Taylor AJ, et al. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol. 2003;4:1. doi: 10.1186/1471-2369-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Folsom AR, Chambless LE, Duncan BB, et al. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care. 2003;26:2777–2784. doi: 10.2337/diacare.26.10.2777. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Kalantar-Zadeh K. Introduction: the reverse epidemiology controversy. Semin Dial. 2007;20:485. doi: 10.1111/j.1525-139X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K. What is so bad about reverse epidemiology anyway? Semin Dial. 2007;20:593–601. doi: 10.1111/j.1525-139X.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- 27.Volkova N, McClellan W, Soucie JM, et al. Racial disparities in the prevalence of cardiovascular disease among incident end-stage renal disease patients. Nephrol Dial Transplant. 2006;21:2202–2209. doi: 10.1093/ndt/gfl078. [DOI] [PubMed] [Google Scholar]
- 28.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi H, Xiang Q, Klein JP. Risk factors for non-fatal myocardial infarction cardiac death in incident dialysis patients. Nephrol Dial Transplant. 2009;24:258–266. doi: 10.1093/ndt/gfn426. [DOI] [PubMed] [Google Scholar]
- 30.Hirth RA, Turenne MN, Wheeler JR, et al. Provider monitoring and pay-for-performance when multiple providers affect outcomes: an application to renal dialysis. Health Serv Res. 2009;44:1585–1602. doi: 10.1111/j.1475-6773.2009.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first”. Hemodial Int. 2009;13:533–542. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 32.Foley RN, Roberts TL, Liu J, et al. Mortality from cancer among US hemodialysis patients, 1995–2005. Am J Nephrol. 2010;31:518–526. doi: 10.1159/000303754. [DOI] [PubMed] [Google Scholar]
- 33.Dalrymple LS, Johansen KL, Chertow GM, et al. Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis. 2010;56:522–530. doi: 10.1053/j.ajkd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 35.Seliger SL. Comorbidity and confounding in end-stage renal disease. Kidney Int. 2010;77:83–85. doi: 10.1038/ki.2009.431. [DOI] [PubMed] [Google Scholar]
- 36.Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med. 2003;168:49–53. doi: 10.1164/rccm.200210-1231OC. [DOI] [PubMed] [Google Scholar]
- 37.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 38.Seliger SL, Gillen DL, Longstreth WT, Jr, et al. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 39.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 40.Boulanger L, Hauch O, Friedman M, et al. Warfarin exposure and the risk of thromboembolic and major bleeding events among medicaid patients with atrial fibrillation. Ann Pharmacother. 2006;40:1024–1029. doi: 10.1345/aph.1G408. [DOI] [PubMed] [Google Scholar]