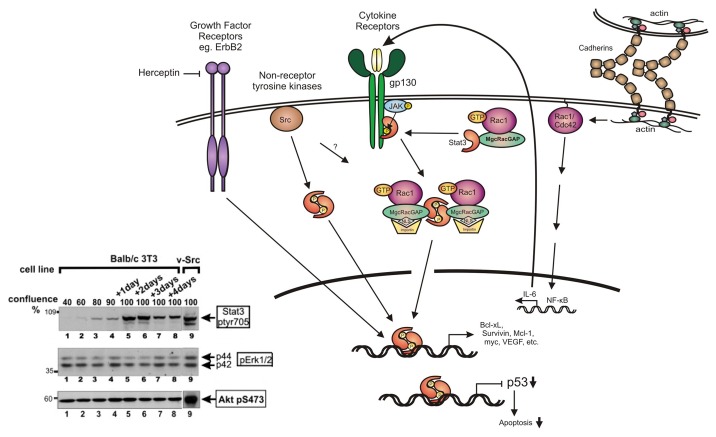
Figure 1. STAT3 activation. Following activation of receptors such as cytokine receptors of the IL-6 family or growth factor receptors such as Her2/ErbB2 (that can be inhibited by drugs such as herceptin), or expression of activated forms of non-receptor tyrosine kinases such as Src, STAT3 is activated by phosphorylation at tyr705 by the receptor itself, or the associated Src or JAK kinases. STAT3 phosphorylation by the IL-6/JAK complex or Src is facilitated through binding to activated Rac1-GTP in a complex with MgcRacGAP. This results in targeting of the complex to the nuclear envelope, driven by the NLS (nuclear localization signal) of MgcRacGAP. The STAT3 dimer then binds specific DNA sequences to initiate transcription of STAT3-responsive genes, or downregulation of other genes such as the p53 tumor suppressor. In addition to this mechanism, cadherin engagement was shown to cause a dramatic increase in the levels of Rac1 and Cdc42 proteins and activity, which results in a transcriptional activation of IL-6 through NFκB, hence STAT3 activation. At the same time, cadherin engagement suppresses Erk1/2 activation by IL-6 (not shown). Inset: Lysates from mouse Balb/c3T3 fibroblasts were grown to different densities as indicated and probed for STAT3-ptyr705, pErk1/2 or active Akt-pser473 (lanes 1–8). Lane 9: Balb/c3T3 cells transformed by activated Src. Note that STAT3-ptyr705 is dramatically increased with density, while levels of p-Erk and Akt-pS473 remain unchanged. Similar results were obtained by plating cells on surfaces coated with E-cadherin or cadherin-11 fragments.19,20 (Modified and adapted from refs. 2 and 20).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.