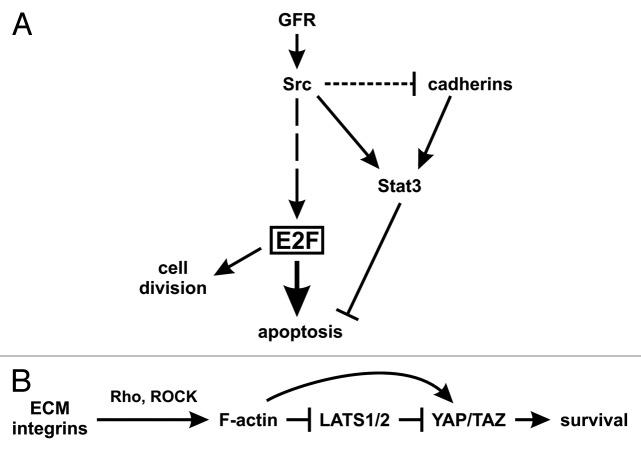
Figure 3. Model of STAT3 as a survival signal. (A) In transformed cells. Growth factor receptors or Src activate the transcription factor E2F1–3a, through a number of steps.58 E2F, in turn, can induce apoptosis through p53-dependent and -independent pathways, while Src or activated receptors also activate STAT3 which blocks apoptosis, so that transformation can occur. However, cadherin engagement may also be necessary to increase STAT3 activity further, even in cells expressing STAT3 activating oncogenes such as Src, and despite the fact that Src may also trigger cadherin downregulation (Guy, Raptis, et al., in preparation). (B) In normal tissues or cells grown to high densities. When cells are spread on the extracellular matrix (ECM), the forces exerted through integrin-ECM adhesion promote the formation of contractile F-actin structures with myosin molecules, regulated by the Rho GTPase. F-actin filaments oppose YAP/TAZ phosphorylation and degradation through inhibition of the LATS kinases and other mechanisms yet to be identified. In confluent cultures or tissues on the other hand, mechanical constraints reduce integrin-ECM adhesion and F-actin formation, thus allowing LATS activation, hence downregulation of YAP/TAZ and apoptosis.1 At the same time, cadherins activate STAT3 to achieve cell survival. As a result, STAT3 inhibition would cause apoptosis in confluent cultures. (Not shown: Cadherin engagement may also lead to the formation of contractile F-actin.)

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.