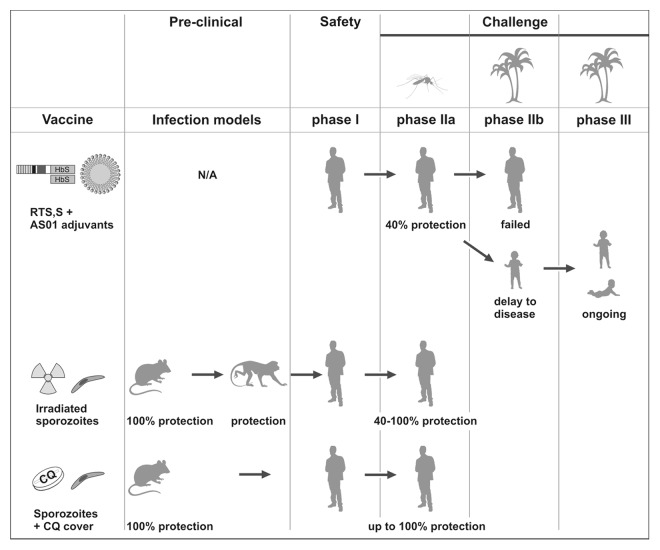
Figure 1. Clinical development of exemplary malaria vaccine candidates. RTS,S/AS01 (top) is currently the clinically most advanced subunit vaccine candidate. The recombinant proteins are shown as boxes (HbS, hepatitis B surface antigen) and the adjuvant AS01 as a lipid droplet. It was developed in the 80s and consistently protected 40% of adult volunteers against homologous challenge with infected laboratory-reared Anopheles mosquitoes (mosquito symbol). In a subsequent field trial (palm symbol) no protection was afforded in adults. Pictograms represent adults, 5–17 mo old children, and 6 weeks to 3 mo old infants. N/A, not applicable. Irradiated sporozoites (center) were first tested in a murine infection model in the 60s and shown to consistently protect non-human primates and adult volunteers against homologous challenge with infected laboratory-reared Anopheles mosquitoes. Sporozoite infection under chloroquine cover (bottom) was tested in a murine infection model in the 70s and recently shown to elicit robust and lasting protection in adult volunteers against homologous challenge with infected laboratory-reared Anopheles mosquitoes.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.