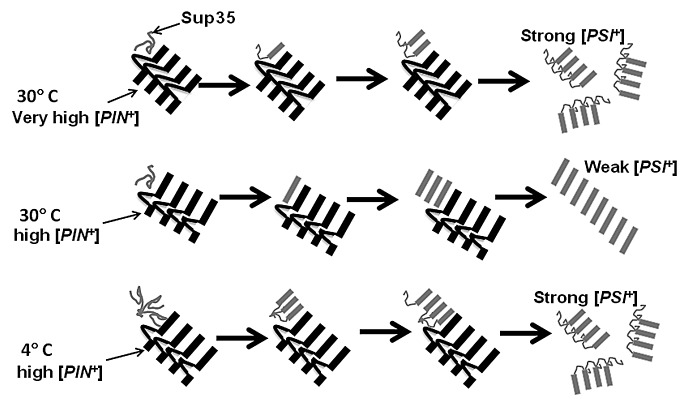
Figure 1. Model showing preferential seeding of specific [PSI+] variants by [PIN+] variants at different temperatures. Very high and high [PIN+] prion domains are cartooned as parallel in register β sheets (black bars) with non-β sheet loops in variant-specific positions. The Sup35 prion domain (gray) is preferentially cross seeded into parallel in register β sheets38 (gray bars) by different lengths of β sheet regions available in high and very high [PIN+]. In the left, Sup35 is shown to first bind to the [PIN+] aggregates (step 1). Only then does the [PIN+] amyloid region convert the Sup35 into amyloid (step 2). Due to the interference of its non-β sheet loop, very high [PIN+] preferentially seeds strong [PSI+], with a small β-sheet core which is vulnerable to sheering and the production of the smaller aggregates characteristic of strong [PSI+]. High [PIN+], which has a larger uninterrupted β-sheet region, preferentially seeds weak [PSI+] with a large β-sheet core. At 4 °C, soluble Sup35 is shown forming a nucleus that interferes with the initial β sheet conversion even when seeded by high [PIN+]. This results in the formation of a smaller β sheet-core and the induction of strong [PSI+]. Evidence for the in vitro formation of such a Sup35 nucleus at 4 °C has been presented.35

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.