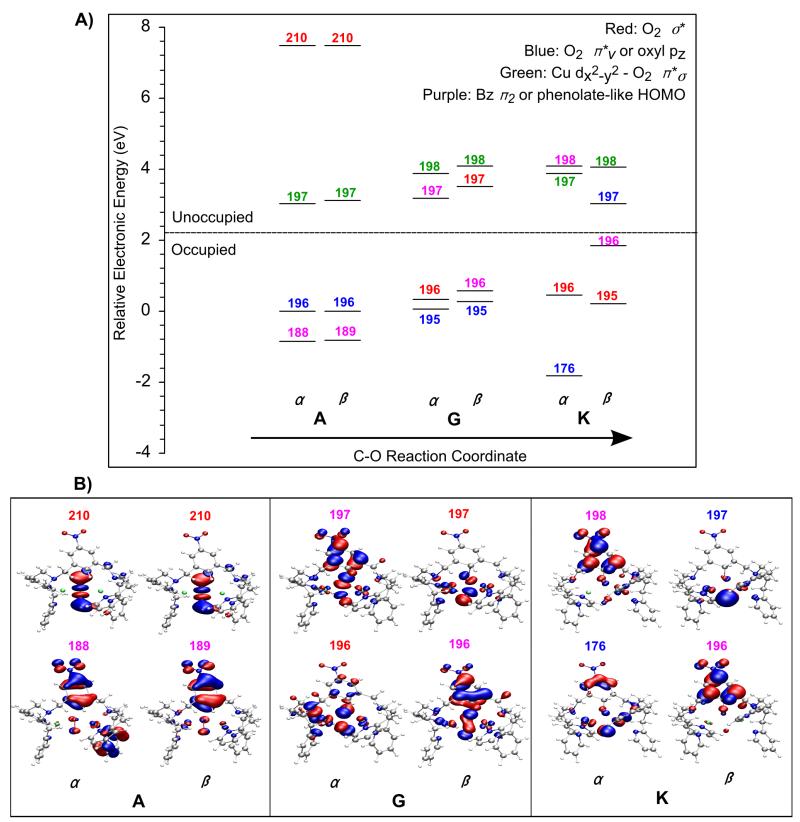
Figure 10.
Key orbitals along the C12–O3 reaction coordinate for [Cu(II)2(NO2-XYL)(O2)]2+. The unoccupied peroxo σ* orbitals (210 α, β in A) are lowered in energy along the coordinate. An α electron is donated from the occupied xylyl HOMO (orbital 188 in A) to the peroxo σ* orbital leaving an α hole on the xylyl (orbital 198 α in K). The β hole ends up on the distal O4 (oxyl pz-like orbital, 197β in K; the z-axis is perpendicular to the Cu2O2 plane).