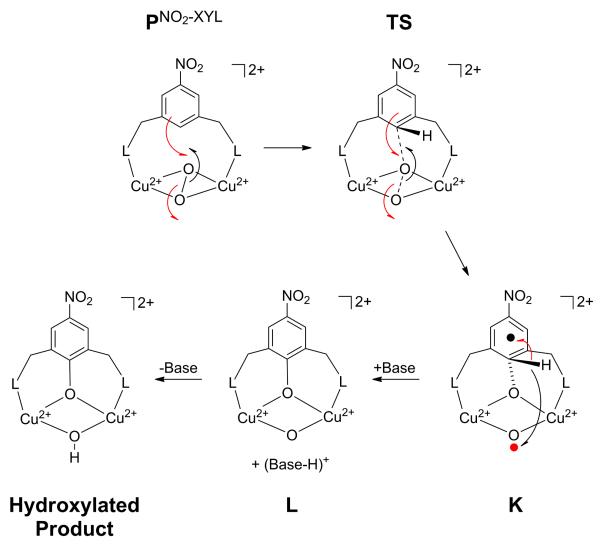
Figure 15.
Schematic mechanism of aromatic hydroxylation performed by PNO2−XYL in [Cu(II)2(NO2-XYL)(O2)]2+. The red and black arrows represent transfer of α and β electrons, respectively. In going through the transition state (TS) to K, a pair of α and β electrons are transferred from the xylyl ring and peroxo π* orbital, respectively, to concertedly form the C-O bond and cleave the O-O bond. This produces an α spin on Od that is paired with the β radical on the ring. In proceeding from K to L and subsequently to the hydroxylated product, the o-H+ is transferred to the Od via a base. As the o-C-H bond is cleaved, its α and β electrons are transferred to the phenolate and Od, respectively, leading to the full formation of the phenolate C-O bond.