SUMMARY
SETTING
Conventional approaches to tuberculosis (TB) diagnosis and resistance testing are slow. The Xpert® MTB/RIF assay is an emerging molecular diagnostic assay for rapid TB diagnosis, offering results within 2 hours. However, the cost-effectiveness of implementing Xpert in settings with low TB prevalence, such as the United States, is unknown.
OBJECTIVE
We evaluated the cost-effectiveness of incorporating Xpert into TB diagnostic algorithms in the United States compared to existing diagnostics.
DESIGN
A decision-analysis model compared current TB diagnostic algorithms in the United States to algorithms incorporating Xpert. Primary outcomes were the costs and quality-adjusted life years (QALYs) accrued with each strategy; cost-effectiveness was represented using incremental cost-effectiveness ratios (ICER).
RESULTS
Xpert testing of a single sputum sample from TB suspects is expected to result in lower total health care costs per patient (US$2673) compared to diagnostic algorithms using only sputum microscopy and culture (US$2728) and improved health outcomes (6.32 QALYs gained per 1000 TB suspects). Compared to existing molecular assays, implementation of Xpert in the United States would be considered highly cost-effective (ICER US$39 992 per QALY gained).
CONCLUSION
TB diagnostic algorithms incorporating Xpert in the United States are highly cost-effective.
Keywords: GeneXpert, MTD, diagnostics
Tuberculosis (TB) is the second most common cause of death due to infectious disease in the world, with over 10 000 cases of active TB disease in the United States.1 Among the challenges in controlling TB is the lack of rapid, accurate diagnostic tests. Currently, sputum smear microscopy is used as the initial test in most diagnostic algorithms, but it has poor sensitivity, leading to missed diagnoses.2 Smear microscopy is also a marker of infectiousness, and current US guidelines suggest isolation of TB suspects with smear-positive results pending results from mycobacterial culture or response to treatment.3–5 However, smear microscopy is not specific to Mycobacterium tuberculosis and has low positive predictive value in low-prevalence settings such as the United States, leading to unnecessary treatment and prolonged hospitalizations. Sputum culture and conventional drug susceptibility testing (DST) are utilized as the reference standard in the United States, but these take weeks to provide results, leading to diagnostic and therapeutic delays.2
The Amplified MTD® (Mycobacterium Tuberculosis Direct) test (Gen-Probe, San Diego, CA, USA), a molecular assay that detects M. tuberculosis genetic material and can provide a rapid diagnosis of TB disease, is Food and Drug Administration (FDA) approved for use in the United States.6,7 For smear-positive samples, the Amplified MTD test has high sensitivity of between 91% and 100% and a high negative predictive value.6–8 It thus allows rapid confirmation of M. tuberculosis in smear-positive samples, and it can reduce the duration of respiratory isolation and prevent empiric medication expenses.6–8 However, its sensitivity on smear-negative samples is approximately 50% and remains suboptimal, and it is not a replacement for mycobacterial culture.3,6–8 The Centers for Disease Control and Prevention nonetheless recommends that at least one respiratory specimen from all TB suspects be sent for molecular testing.7 Despite these recommendations, however, broad implementation of molecular testing remains limited, as they are labor- and resource-intensive. In our local setting, for example, few hospital laboratories perform MTD, and the state mycobacteriology reference laboratory performs routine MTD testing only for smear-positive samples.3
Improved molecular TB diagnostic systems are now commercially available that are faster and require less labor than MTD, with improved performance characteristics. The Xpert® MTB/RIF test (Cepheid Inc, Sunnyvale, CA, USA) is an automated nucleic-acid amplification test for the diagnosis of TB, offering results in 2 h. Importantly, Xpert requires minimal laboratory equipment, space and technician time and also provides rapid identification of rifampin resistance, allowing earlier treatment of drug-resistant TB. Studies have found sensitivity and specificity for TB and drug resistance to be >97% on smear-positive samples, while sensitivity on smear-negative samples may be as high as 70–80%.9,10 It was therefore endorsed by the World Health Organization (WHO) for the detection of pulmonary TB.11 Although Xpert is not yet FDA-approved in the United States, it may be implemented in US laboratories after appropriate internal laboratory validations, with results reported with a disclaimer.
Several mycobacterial laboratories in the United States are currently considering the adoption of Xpert, but its cost-effectiveness in low TB prevalence settings is unknown. In low-income settings globally, Xpert is available at a negotiated discounted price; implementation in such settings with high TB incidence was found to be cost-effective.12–14 However, the United States does not qualify for reduced Xpert pricing and its optimal role in existing diagnostic algorithms is unclear. We thus sought to evaluate the cost-effectiveness of incorporating Xpert into TB diagnostic algorithms compared to current approaches in the United States.
METHODS
This economic evaluation was conducted from a health system perspective with a target population of individuals with suspected pulmonary TB disease in the United States. Target audiences include health departments, hospitals and TB control programs. A timeframe of 1 year was used and the analytic horizon extended to the life expectancy of the patients. Model development and analysis utilized TreeAge Software (TreeAge Software Inc, Williamstown, MA, USA).
Study model
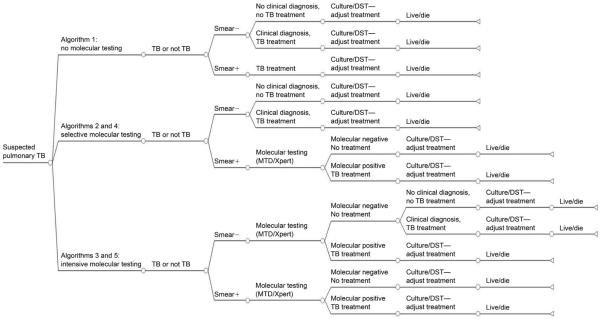
A decision-analysis model was constructed to determine if TB diagnostic algorithms that incorporate Xpert are cost-effective compared to current TB diagnostic strategies using MTD or without any molecular testing (Figure 1). In all model arms, patients submit three sputum samples for mycobacterial testing and undergo a chest radiograph and clinical evaluation; mycobacterial testing (i.e., `conventional diagnostics') includes smear microscopy and liquid culture, which are performed on all sputum samples, and DST performed on positive cultures. Treatment was assumed to be initiated and/or adjusted based on diagnostic test results. We compared five strategies for pulmonary TB diagnosis with and without the incorporation of molecular testing (the model details are described in Appendix A).*
Algorithm 1: a `no molecular testing' algorithm, in which sputum samples are sent for conventional diagnostics; no molecular tests are employed.
Algorithm 2: a `selective MTD' algorithm, in which sputum samples are sent for conventional diagnostics; MTD testing is selectively performed on one sample only if smear microscopy is positive.
Algorithm 3: an `intensive MTD' algorithm, in which sputum samples are sent for conventional diagnostics; MTD testing is performed on one sample, regardless of smear microscopy results.7
Algorithm 4: a `selective Xpert' algorithm, in which sputum samples are sent for conventional diagnostics; Xpert testing is selectively performed on one sample only if smear microscopy is positive.
Algorithm 5: an `intensive Xpert' algorithm, in which sputum samples are sent for conventional diagnostics; Xpert testing is performed on one sample, regardless of smear microscopy results.
Figure 1.
Schematic diagram of decision-analysis model. Not all branches are shown; model details are found in Appendix A. `Selective' molecular algorithms (Algorithms 2 and 4) incorporate molecular testing with either Xpert® MTB/RIF or MTD® for sputum samples positive by smear microscopy, but not for sputum samples that are smear-negative. `Intensive' molecular algorithms incorporate molecular testing of at least one sputum, regardless of sputum microscopy results. All algorithms include testing of three sputum samples by conventional diagnostics. TB = tuberculosis; − = negative; + = positive; DST = drug susceptibility testing.
Epidemiologic and diagnostic parameters
Data regarding disease prevalence and diagnostic test performance are summarized in Table 1.
Table 1.
Model parameters and sources
| Variable | Base case | Low | High | Reference |
|---|---|---|---|---|
| Laboratory costs, US$* | ||||
| Smear microscopy | 4.07 | 2.35 | 5.95 | Calculated |
| Mycobacterial culture | 35.56 | 17.29 | 52.60 | Calculated |
| DST | 101.68 | 19.60 | 166.37 | Calculated |
| MTD® | 91.49 | 26.08 | 320.42 | Calculated |
| Xpert® MTB/RIF | 98.10 | 20.24 | 838.46 | Calculated |
| Treatment costs, US$ | ||||
| Anti-tuberculosis treatment course (drug-susceptible TB, 6 months) | 9 037† | 3 069 | 53 401 | BCHD†, 15, 16 |
| Hospitalization costs per day | 2 469 | 1 161 | 2 975 | 17, 18 |
| MDR-TB treatment | 57 889† | 40 133 | 204 862 | BCHD†, 15, 19 |
| Epidemiology and diagnostic and treatment parameters | ||||
| Prevalence of TB among TB suspects in the United States (HIV-positive TB suspects)§ | 0.02 (0.062) | 0.01 (0.03) | 0.30 (0.30) | 1, 20, 21‡ |
| MDR-TB prevalence among TB cases in the United States | 0.011 | 0.009 | 0.075 | 1 |
| Probability of hospitalization during initial TB evaluation | 0.20 | 0 | 1.0 | BCHD, 15, 22 |
| Mortality of untreated smear-positive TB (smear-negative TB)¶ | 0.70 (0.20) | 0.53 (0.10) | 0.86 (0.30) | 23, 24 |
| Mortality of treated drug-susceptible TB (MDR-TB)¶ | 0.05 (0.08) | 0.01 (0.05) | 0.20 (0.50) | 1 |
| Sensitivity, smear microscopy (HIV-positive TB suspects) | 0.81 (0.25) | 0.45 (0.20) | 0.90 (0.50) | 25, 26 |
| Specificity, smear microscopy | 0.97 | 0.91 | 1.0 | 25, 26 |
| Sensitivity, mycobacterial culture for smear-positive TB (smear-negative TB) | 1.0 (0.9) | 0.90 (0.70) | 1.0 (1.0) | Assumption |
| Sensitivity, MTD for smear-positive TB (smear-negative TB) | 0.98 (0.55) | 0.91 (0.45) | 1.0 (0.90) | 6–8, 27 |
| Specificity, MTD | 0.99 | 0.95 | 1.0 | 6–8, 27 |
| Sensitivity, clinical diagnosis (specificity) | 0.3 (0.9) | 0 (0.80) | 0.80 (1.0) | 1, 14 |
| Sensitivity, Xpert MTB/RIF for smear-positive TB (smear-negative TB) | 0.98 (0.72) | 0.95 (0.50) | 1.0 (0.90) | 9, 14 |
| Specificity, Xpert MTB/RIF | 0.99 | 0.95 | 1.0 | 9, 14 |
| Sensitivity, Xpert MTB/RIF rifampin resistance detection (specificity) | 0.976 (0.98) | 0.90 (0.90) | 1.0 (1.0) | 9, 14 |
| Sensitivity, conventional DST resistance detection (specificity) | 1.0 (1.0) | — | — | Assumption |
| Utility weight | ||||
| Complete health | 1 | 16 | ||
| First-line treatment# | 0.9 | 0.7 | 0.95 | 16 |
| MDR-TB treatment# | 0.7 | 0.5 | 0.95 | 16, Assumption |
| Treated active TB disease** | 0.85 | 0.7 | 0.9 | 16, 28 |
| Untreated active TB disease** | 0.7 | 0.5 | 0.9 | 16, 28 |
| Drug-related hepatotoxicity | 0.8 | 0.7 | 0.95 | 16 |
| Death | 0 | 16 |
Cost of component consumables and equipment were obtained from manufacturers or distributors. Quantity of consumables and equipment as well as labor and overheads were determined through direct observation at the Johns Hopkins Hospital Mycobacteriology Laboratory and the Maryland DHMH Mycobacteriology Laboratory. Detailed breakdown of costs is shown in Appendix B.
The base case cost of anti-tuberculosis treatment was calculated from Baltimore City Health Department records and invoices and reflects the costs of out-patient care. The base case costs include labor, drugs and diagnostic monitoring, and assume no additional hospitalizations during treatment. Labor associated with clinicians, nurses, case managers, and directly observed therapy accounted for US$8313 (92%) of costs. For MDR-TB, costs included those associated with extended treatment course, second-line drug regimens, and estimated in-patient hospitalizations during treatment. Range was determined by using lowest and highest estimates for labor, drugs, diagnostic monitoring costs, and complications and hospitalization during treatment.
Kim Dionne, personal communication, Johns Hopkins Hospital Mycobacteriology Laboratory, Baltimore, MD, USA, 2012.
In the base case, we assumed 7% of all TB suspects were HIV-infected, and varied this between 5% and 20% in sensitivity analysis.
Parameters were varied for HIV infection. For untreated smear-positive TB, mortality was assumed to be 83%; for smear-negative TB, 74%. For treated drug-susceptible TB, base case mortality was assumed to be 13%; for MDR-TB, 18%.24
Represents utility weight associated with being placed on medication regimen and is independent of TB disease utility.
We assumed that the severity of disease was the same for drug-susceptible and drug-resistant TB, but the duration of disease differed between the two. We assumed that average treatment duration for drug-susceptible TB was 6 months and treatment duration for MDR-TB was 18 months.
DST = drug susceptibility testing; TB = tuberculosis; BCHD = Baltimore City Health Department; MDR-TB = multidrug-resistant TB; HIV = human immunodeficiency virus; DHMH = Department of Health & Mental Hygiene.
Estimation of costs
Costs for diagnostics are shown in Table 1, with additional details in Appendix B.29 The amount of staff time, consumable supplies and equipment utilized for each test system were determined through direct observation of testing procedures at the Maryland Department of Health and Mental Hygiene Mycobacteriology Laboratory and the Johns Hopkins Hospital Mycobacteriology Laboratory. Costs of key consumable items and equipment for each diagnostic test were obtained from manufacturer/distributor quotations and published literature; salaries and wages were based on published estimates.8 For the base case, we utilized published estimates from the manufacturer for commercial pricing of Xpert cartridges (US$71.63 per cartridge) and 4-cartridge Xpert instrument (US$78 200).13
Little has been published on TB treatment costs in the United States.15 We utilized local health department invoices and budget records to determine out-patient costs of TB treatment and monitoring, including staff labor, directly observed therapy and medications.30 For the base case, we assumed that 20% of TB suspects were initially evaluated as in-patients, and this included initial hospitalization costs. We conducted sensitivity analysis on all key cost parameters. Costs are presented in 2012 US dollars.
Outcome parameters
The primary outcomes were the expected costs per patient with suspected pulmonary TB, quality-adjusted life years (QALYs) accrued per patient, and the cost-effectiveness of the proposed diagnostic algorithms. Cost-effectiveness was represented using incremental cost-effectiveness ratios (ICERs) comparing new TB diagnostic algorithms incorporating Xpert with standard approaches without molecular diagnostics or algorithms incorporating MTD. QALY calculations were based on duration with and without active TB and/or anti-tuberculosis treatment; future QALYs were discounted at 3% (Table 1).
We further explored parameter uncertainty through probabilistic sensitivity analysis using Monte-Carlo simulation methods. We utilized a willingness-to-pay (WTP) threshold of US$50 000 per QALY gained to determine cost-effectiveness, and explored other thresholds using cost-effectiveness acceptability curves.11,31
Ethics statement
This economic evaluation was approved by the ethics committee at the Johns Hopkins University School of Medicine (Baltimore, MD, USA) as exempt and did not constitute human subjects research.
RESULTS
Laboratory and health system costs for tuberculosis diagnostic algorithms
Incremental laboratory costs of each diagnostic algorithm are shown in Table 2. In the base case, laboratory costs for an algorithm without molecular testing were US$158 per patient with suspected pulmonary TB (Algorithm 1). Implementing `intensive' Xpert testing (Algorithm 5) for at least one sputum sample increases laboratory costs to US$256 per patient (incremental US$98 [62%] increase). `Selective' deployment of Xpert only for smear-positive samples is cheaper than `intensive' molecular testing and costs US$162 per patient (Table 2).
Table 2.
Costs and cost-effectiveness of diagnostic algorithms
| Costs and effects | Algorithm 1 no molecular | Algorithm 2 selective MTD® | Algorithm 3 intensive MTD® | Algorithm 4 selective Xpert® | Algorithm 5 intensive Xpert® |
|---|---|---|---|---|---|
| Total laboratory costs per TB suspect, US$ | 157.64 | 161.80 | 249.13 | 162.10 | 255.75* |
| Incremental laboratory costs, US$ | Reference | 4.16 | 91.49 | 4.46 | 98.11 |
| Total health care costs per TB suspect, US$† | 2727.68 | 2479.63 | 2653.08 | 2481.71 | 2672.79 |
| Incremental total costs, US$ | Reference | −248.05 | −74.60 | −245.97 | −54.89 |
| QALYs accrued per TB suspect | 22.08622 | 22.08771 | 22.09133 | 22.08780 | 22.09254 |
| Incremental QALYs per 1000 suspects | Reference | 1.49 | 5.11 | 1.58 | 6.32‡ |
| Average time to diagnosis among TB cases, days§ | 16.30 | 13.31 | 3.92 | 6.03 | 2.71 |
| TB cases diagnosed by molecular testing, % | 0 | 75.50 | 88.00 | 75.50 | 92.00 |
| ICER, US$ per QALY gained¶ | Dominated¶ | Reference | 47 914 | 23 111 | 39 992 |
| ICER, US$ per QALY gained | — | — | Reference | — | 16 289 |
| ICER, US$ per QALY gained | — | — | — | Reference | 40 312 |
The base case scenario assumes a volume of testing of 3000 TB suspects per year. If the volume of testing is reduced to a low estimate of 150 TB suspects per year, laboratory costs per suspect for `intensive' Xpert algorithm increase to $384 per TB suspect. Total laboratory costs include the cost of smear microscopy and culture for three samples per TB suspect and Xpert testing of one sample, as well as costs associated with preparation, clean-up and reporting.
Total health care costs include costs associated with initial diagnostic evaluations, including laboratory testing, hospitalization, respiratory isolation, as well as costs associated with anti-tuberculosis treatment (includes labor, drugs and monitoring). For the base case, we assumed that 20% of TB suspects receive initial diagnostic evaluation in the in-patient setting.
`Intensive' Xpert (Algorithm 5) compared with `selective' Xpert (Algorithm 4) testing resulted in incremental 4.74 QALYs gained per 1000 TB suspects; `intensive' Xpert MTB/RIF (Algorithm 5) compared to intensive MTD (Algorithm 3) testing resulted in 1.21 QALYs gained per 1000 TB suspects.
Time to diagnosis is based on estimated time to earliest correct diagnosis. We estimated that smear microscopy and Xpert results are available within 1 day, and average time to culture positivity was 16 days; algorithms with selective implementation of molecular assays assumed sequential testing with molecular assay after smear microscopy results are available.
Algorithm 1 was dominated by all other algorithms (i.e., was more costly and less effective). ICERs were calculated as incremental costs divided by incremental effects.
TB = tuberculosis; QALY = quality-adjusted life year; ICER = incremental cost-effectiveness ratio.
When all health system costs are considered, a strategy without molecular testing was found to be the most costly approach ($2728 per patient, Algorithm 1). Molecular testing with MTD or Xpert either `selectively' or `intensively' (Algorithms 2–5) results in less empiric treatment and shorter hospitalizations, and were subsequently less costly ($2480 to $2673 per patient) than a strategy without molecular testing (Table 2). Overall, `intensive' Xpert testing ($2673 per patient) was expected to cost US$191 more (8% increase in total costs) than a `selective' strategy of Xpert testing of only smear-positive samples ($2482 per patient).
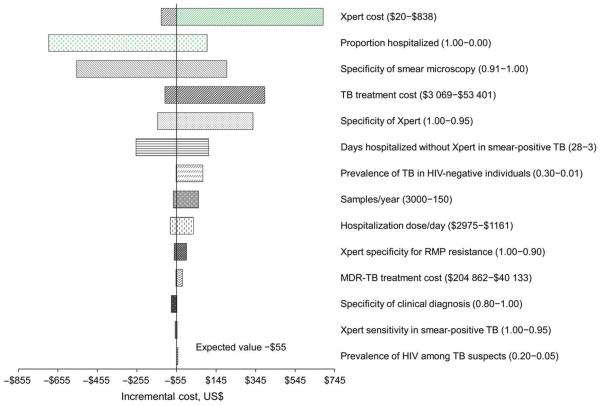
In one-way sensitivity analysis, the incremental cost of `intensive' Xpert implementation compared to an algorithm without molecular testing was most influenced by the cost of Xpert (incremental −$133 [cost-saving] to $685 for costs per test ranging from $20 to $838), the percentage of patients hospitalized for initial evaluation (incremental −$702 to $106 for percentage hospitalization ranging from 100% to 0%) and specificity of smear microscopy (incremental −$561 to $198 for specificity 91% to 100%, Appendix Figure C.1).
Effects
Overall, a strategy without molecular testing (Algorithm 1) resulted in the fewest QALYs experienced (22.0862) per patient, while `intensive' Xpert testing resulted in the most QALYs experienced per patient (22.0925 QALYs; incremental 6.32 QALYs gained per 1000 patients, Table 2). Xpert testing was expected to reduce the time to diagnosis for TB cases with subsequent QALY gains (Table 2, estimated time to diagnosis reduced from 16.3 days without molecular testing to 2.7 days with `intensive' Xpert testing among those with TB). `Intensive' Xpert testing was associated with an incremental increase of 4.74 QALYs gained per 1000 TB suspects compared with `selective' Xpert testing (Table 2).
In one-way sensitivity analyses, there were no circumstances in which a diagnostic algorithm without molecular testing (Algorithm 1) led to better health outcomes than strategies incorporating molecular testing (Algorithms 2–5, Appendix Figure C.2).
Cost-effectiveness
A strategy of no molecular testing (Algorithm 1) was dominated (i.e., was both more costly and less effective) by all strategies incorporating either MTD or Xpert for TB diagnosis (Table 2). Replacing MTD with Xpert was also found to be cost-effective. Compared to `selective' MTD testing of only smear-positive sputum samples (Algorithm 2), utilizing Xpert `selectively' was associated with an ICER of only $23 111 per QALY-gained (Table 2), and was considered highly cost-effective compared to the WTP for the United States.32
The `intensive' Xpert algorithm was associated with an ICER of $16 289 per QALY gained compared to the `intensive' MTD algorithm. For programs considering `selective' vs. `intensive' Xpert implementation, `intensive' Xpert testing was highly cost-effective compared to the `selective' Xpert algorithm (ICER of $40 312 per QALY-gained, Table 2).
In one-way sensitivity analysis, we found that the `intensive' Xpert algorithm dominated (i.e., negative ICER) the algorithm without molecular testing (Algorithm 1) in most circumstances; `intensive' Xpert was cost-effective at the WTP in all scenarios except when cost per Xpert test rose above $475 or Xpert specificity was lower than 96%. For out-patient evaluations, `intensive' Xpert was cost-effective compared to Algorithm 1 (ICER US$16 900 per QALY gained); by contrast, for in-patient evaluations, `intensive' Xpert dominated Algorithm 1. Additional sensitivity analysis comparing the `intensive' Xpert algorithm with `selective' molecular algorithms is shown in Appendix Figures C.3 and C.4.
Results of a probabilistic sensitivity analysis (shown in Figure 2 and Appendix D) found that the `intensive' Xpert algorithm was cost-effective in more than 99% of simulations compared to diagnostic algorithms without molecular testing.
Figure 2.
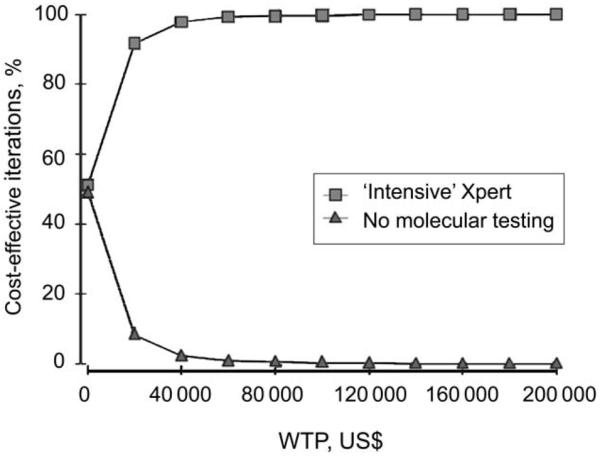
Cost-effectiveness acceptability curve comparing Algorithm 1 (no molecular testing) vs. Algorithm 5 (`intensive Xpert'). Cost-effectiveness acceptability curve showing the probability that implementation of an `intensive' Xpert MTB/RIF diagnostic algorithm (Algorithm 5) will be cost-effective compared to an algorithm without molecular testing (Algorithm 1) at varying thresholds WTP. At WTP of $50K, `intensive Xpert' was cost-effective in 99% of simulations; at WTP $100K, `intensive Xpert' was cost-effective in 99.8% of simulations. WTP = willingness-to-pay.
DISCUSSION
In the United States, diagnostic testing for pulmonary TB includes sputum smear microscopy and mycobacterial culture, but can lead to diagnostic delays and inappropriate empiric anti-tuberculosis treatment. Following the WHO endorsement of the Xpert assay for the rapid detection of TB and drug-resistant TB, several low- and middle-income countries with a high TB burden have taken advantage of negotiated price reductions to scale up Xpert implementation.11,33 Although the costs of Xpert testing are likely to be substantially higher in the United States, we show that the incorporation of Xpert into TB diagnostic algorithms in the United States would be highly cost-effective compared to current algorithms that utilize conventional diagnostics and existing molecular assays. From a health system standpoint, we found that the implementation of Xpert testing as an addition to microscopy and culture is expected to be less costly and more effective (i.e., cost-saving) than diagnostic algorithms that rely on sputum microscopy and culture alone. Some programs have chosen to implement molecular assays selectively only for smear-positive sputum samples;3 however, we found that `intensive' implementation of Xpert for at least one sputum sample from all individuals with signs/symptoms of TB was highly cost-effective compared to the more selective approach.
Despite the availability of mycobacterial culture as the reference standard in current US diagnostic algorithms, the addition of rapid Xpert testing leads to a gain in QALYs experienced by patients as a result of more rapid diagnosis and treatment of active TB, and less unnecessary treatment in cases of false-positive smear microscopy. Moreover, we found that the use of `intensive' Xpert testing was associated with increased health utilities compared with use of the currently FDA-approved molecular assay (i.e., MTD) or `selective' use of molecular assays only for smear-positive samples.
From a laboratory perspective, Xpert testing of at least one sputum sample would increase costs by over 60% per patient compared to no molecular testing. Despite the higher laboratory costs, however, we found that incorporating Xpert into diagnostic algorithms in the United States would be cost-saving from a health systems perspective. These cost savings are partially attributable to the low positive predictive value of smear microscopy in a low-prevalence setting, which can lead to increased health care expenditures while awaiting mycobacterial culture results.
Our study has several limitations. First, we did not include the potential costs and effects associated with TB transmission that may occur during diagnostic delays. Despite this, we found that Xpert implementation was cost-effective compared to conventional approaches; incorporation of transmission would be expected to further enhance cost-effectiveness. Second, the costs of Xpert testing in the United States are not yet well defined and may vary across laboratories and hospitals. Nonetheless, we conducted extensive sensitivity analysis and found that Xpert implementation would be cost-effective even at higher Xpert test costs. Interestingly, laboratory costs for countries that qualify for prices negotiated by the Foundation for Innovative New Diagnostics (Geneva, Switzerland) are as little as $20–30 per Xpert test;14,34,35 whether public health laboratories in the United States will qualify for lower negotiated rates in the future remains unknown. Finally, to allow generalizability, our analysis incorporated both in-patients and out-patients. However, in sensitivity analysis, we found that health system costs of incorporating molecular testing were influenced by hospitalization status. For hospitalized patients, utilizing molecular assays is highly cost-saving due to the reduced need for respiratory isolation and shorter hospitalizations for individuals with false-positive smear microscopy. On the other hand, for out-patients, the use of molecular assays was associated with incremental cost increases. Nevertheless, given the health benefits associated with rapid diagnosis, we show that Xpert implementation would be cost-effective for both in-patient and out-patient settings.
On the other hand our study has several strengths. We are among the first to evaluate the cost-effectiveness of Xpert testing in diagnostic algorithms in a low-prevalence setting that does not qualify for the reduced Xpert pricing available in other parts of the world. We show that despite the availability of mycobacterial culture, the addition of Xpert for TB diagnosis would be beneficial and cost-effective compared to current approaches. Finally, our study is unique in attempting to determine the optimal role for molecular testing in a low-prevalence setting by evaluating multiple algorithms with either `selective' or `intensive' Xpert implementation.
CONCLUSION
The diagnosis of TB disease remains challenging and resource-intensive. We show that Xpert implementation is cost-effective for the diagnosis of pulmonary TB in the United States.
Acknowledgements
The project was supported by a National Institutes Health K23 grant (AI089259) to study novel TB diagnostics. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
APPENDIX A: MODEL DETAILS
Algorithm details
Algorithm 1: No molecular testing
All tuberculosis (TB) suspects submit three sputum specimens for mycobacterial testing. Specimens are tested using smear microscopy and culture, and drug susceptibility testing (DST) if culture-positive. Individuals with a positive sputum smear are initiated on standard treatment per current guidelines.3,5 Individuals with a negative sputum smear result are initiated on treatment if clinical suspicion is high (i.e., clinical diagnosis). For all individuals, treatment is adjusted based on final mycobacterial culture and DST results (i.e., initiated/continued for positive cultures, and discontinued for negative cultures). For hospitalized patients with positive sputum smear results, respiratory isolation is continued for a minimum of 14 days after treatment initiation per current guidelines;3,5 hospitalized patients with negative sputum smear results are assumed to be eligible for discontinuation of respiratory isolation and discharge.
Algorithm 2: Selective Amplified Mycobacterium Tuberculosis Direct
All TB suspects submit three sputum specimens for mycobacterial testing. Specimens are tested using smear microscopy and culture, and if the smear is positive, one specimen is tested using the Amplified MTD® (Mycobacterium Tuberculosis Direct) nucleic acid amplification assay (Gen-Probe, San Diego, CA, USA). A positive sputum smear with a positive MTD result is considered to be a confirmed TB diagnosis and the patient is treated with the standard regimen per current guidelines.3,5 Individuals with a positive sputum smear result with negative MTD testing were assumed to have no treatment initiated. Individuals with a negative sputum smear result are initiated on treatment if clinical suspicion is high (i.e., clinical diagnosis). For all individuals, treatment is adjusted based on final mycobacterial culture and DST results. For hospitalized patients with positive sputum smear results with negative MTD testing, respiratory isolation is discontinued and patient is considered eligible for discharge; hospitalized patients with negative sputum smear results are considered eligible for the discontinuation of respiratory isolation and discharge.
Algorithm 3: Intensive MTD
Algorithm 3 is similar to the `selective' MTD algorithm, except that MTD is performed on one sputum sample of all TB suspects, regardless of smear microscopy results. Positive sputum smears with a positive MTD result are considered to be a confirmed TB diagnosis and the patient is treated with the standardized regimen. Individuals with a positive sputum smear result with a negative MTD result were assumed to have no treatment initiated. Individuals with negative smears with a positive MTD result are started on the standardized TB regimen per current guidelines.3,5 If the smear and MTD are negative, treatment is initiated if clinical suspicion is high (i.e., clinical diagnosis). For all individuals, treatment is adjusted based on final mycobacterial culture and DST results. For hospitalized patients with positive sputum smear results with negative MTD testing, respiratory isolation is discontinued and the patient is considered eligible for discharge; hospitalized patients with negative sputum smear results are considered eligible for discontinuation of respiratory isolation and discharge.
Algorithm 4: Selective Xpert
The diagnostic and clinical approach is the same as for the `selective' MTD algorithm, except that Xpert® MTB/RIF is incorporated into the algorithm in lieu of the MTD test. All TB suspects submit three sputum specimens for mycobacterial testing. Specimens are tested using smear microscopy and culture, and if the smear is positive, one specimen is tested using Xpert. Treatment and clinical care is initiated as outlined in the `selective' MTD algorithm. Xpert also provides rapid DST data when it is performed. We assumed that individuals with positive Xpert results are initiated on standardized treatment regimens for drug-susceptible or drug-resistant TB (based on Xpert results) per current guidelines.3,5 If DST results are not in line with Xpert results, we assumed treatment was adjusted based on conventional DST when these results became available (performed on positive mycobacterial culture isolates).
Algorithm 5: Intensive Xpert MTB/RIF
The diagnostic and clinical approach is the same as for the `intensive' MTD algorithm, except that Xpert is incorporated into the algorithm in lieu of the MTD test. All TB suspects submit three sputum specimens for mycobacterial testing. All specimens are tested by smear microscopy and culture, and one specimen is tested using the Xpert assay, regardless of smear microscopy results. Treatment and clinical care is initiated as outlined in the `intensive MTD' algorithm. Xpert also provides rapid DST data when it is performed. We assumed that individuals with positive Xpert results are initiated on standardized treatment regimens for drug-susceptible or drug-resistant TB (based on Xpert test results) per current guidelines.3,5 If DST results did not correspond to Xpert results, we assumed treatment was adjusted based on conventional DST when these results became available (performed on positive mycobacterial culture isolates).
Additional model assumptions and details
All patients diagnosed with TB on the basis of diagnostic testing are assumed to receive complete treatment with directly observed therapy (DOT), with negli gible rates of non-adherence. For the base case, hospitalized TB suspects with positive sputum smear microscopy were assumed to require a minimum of 14 days of hospitalization with respiratory isolation before discharge per current guidelines;3,5 individuals with sputum smear negativity and/or negative rapid molecular testing were assumed to have the duration of their hospitalization shortened to 3 days.3,5,8 For drug-susceptible TB or when DST results are unavailable, anti-tuberculosis treatment comprised an initial phase of isoniazid (INH), rifampin (RMP), pyrazinamide (PZA) and ethambutol (EMB) for 2 months, followed by a continuation phase of INH and RMP for an additional 4 months.3,5 If multidrug-resistant TB (MDRTB) was detected using conventional DST or molecular testing (RMP resistance on Xpert was assumed to be a marker of MDR-TB), MDR-TB treatment was assumed to consist of an injectable (amikacin), moxifloxacin (MFX), PZA, ethionamide (ETH) and cycloserine (CS) daily for 6 months, followed by 12 months of MFX, PZA, ETH and CS.3,5 Treatment efficacy was based on published estimates and Baltimore City Health Department records.1 The degree to which diagnostic delays impact TB-associated mortality is unknown; for the base case, we assumed a modest 20% relative increase in mortality (absolute increase of 1%) in TB patients without rapid diagnosis/treatment compared to those with rapid diagnosis/treatment and varied this parameter in sensitivity analysis (baseline TB mortality 5%, Table 1).
To estimate the time to diagnosis, we assumed that smear microscopy and Xpert results would be available within 1 day; when molecular tests were performed selectively for smear-positive samples only, we added time for sequential testing. We assumed that the mean time until culture positivity using liquid culture was 16 days, that negative cultures were held until 60 days, that DST took on average of 14 days after isolate grew in culture and that MTD test results would be available within 2 days; diagnostic times were based on discussions with local laboratory managers and based on the literature. We assumed that clinical diagnoses to initiate treatment in the absence of positive test results were made at 14 days after presentation. Given that all algorithms incorporated multiple test results, we used the earliest time to correct diagnosis (for arms with selective molecular testing, we assumed smear-positive cases had diagnostic decisions based on molecular test results); in cases when an incorrect diagnosis was made, we utilized the time until the earliest test results upon which diagnostic decisions were based. TB transmission and secondary cases were not considered in this model.
APPENDIX B
COSTS OF DIAGNOSTIC TESTS PER SAMPLE
| Diagnostic test | Cost
of consumables US$ (% total) |
Cost
of equipment US$ (% total) |
Labor cost US$ (% total) |
Overhead cost US$ (% total)* |
Total cost per sample [range]† |
|---|---|---|---|---|---|
| Decontamination/ concentration |
4.93 (66) | 0.17 (2) | 1.70 (23) | 0.68 (9) | 7.48 [2.58–12.88] |
| Smear microscopy | 0.92 (23) | 0.09 (2) | 2.69 (66) | 0.37 (9) | 4.07 [2.35–5.95] |
| MGIT | 15.02 (42) | 2.87 (8) | 14.16 (40) | 3.51 (10) | 35.56 [17.29–52.60] |
| DST | 57.00 (56) | 23.43 (23) | 11.99 (12) | 9.26 (9) | 101.68 [19.60–166.37] |
| MTD® | 70.37 (77) | 1.50 (2) | 11.30 (12) | 8.32 (9) | 91.49 [26.08–320.42] |
| Xpert® MTB/RIF | 74.60 (76) | 13.94 (14) | 4.78 (5) | 4.78 (5) | 98.10 [20.24–838.46]‡ |
Overhead was assumed to be between 5% and 10% of total costs for each test system based on discussions with laboratory managers.
Laboratory testing capacity was estimated based on laboratory records; for reference laboratories we assumed that 3000 TB suspects are evaluated each year providing 9000 sputum samples. Low and high estimates based on estimated range of vendor pricing for consumables and equipment, variations in laboratory wages and volume of testing (low of 150 TB suspects per year, high of 3000 TB suspects per year); when component estimates were unavailable, unit costs were adjusted by ±75%. Baseline laboratory technician wages were estimated to be $25.47 per hour (range $18.75–$31.80).8
Includes costs associated with indeterminate test results requiring repeat testing, as well as costs associated with instrument calibration and maintenance; we incorporated costs for two 4-cartridge Xpert instruments (Cepheid, Sunnyvale, CA, USA) based on base-case volume. Xpert instrument cost was estimated to be $78200, with Xpert cartridge costs (per test) of $71.36 based on current manufacturer estimates.12, 14 Low and high range of costs determined by using lowest and highest estimates for Xpert consumable costs, Xpert equipment costs, labor costs and volume of testing.
MGIT = Mycobacteria Growth Indicator Tube; DST = drug susceptibility testing.
APPENDIX C: SENSITIVITY ANALYSIS OF COSTS AND EFFECTS
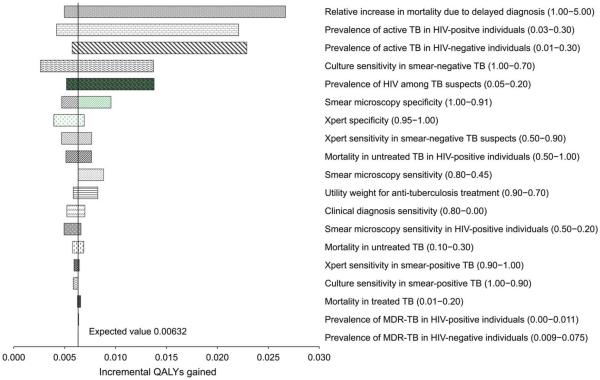
The incremental quality-adjusted life years (QALYs) gained comparing `intensive' Xpert testing to no molecular testing were most influenced by: 1) the relative increase in probability of death with delayed diagnosis (incremental QALYs gained range from 4.9 per 1000 TB suspects if there is no increase in risk of death with diagnostic delay, to 26.7 QALYs gained per 1000 TB suspects if there is a five-fold i ncrease in risk of death with diagnostic delay), and 2) the prevalence of TB among TB suspects (incremental QALYs gained range from 5.7 per 1000 TB suspects to 22.9 per 1000 TB suspects for TB prevalence of 1% to 30% among TB suspects; Figure C.2).
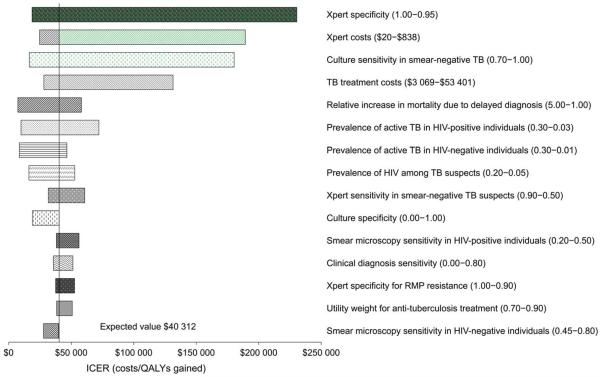
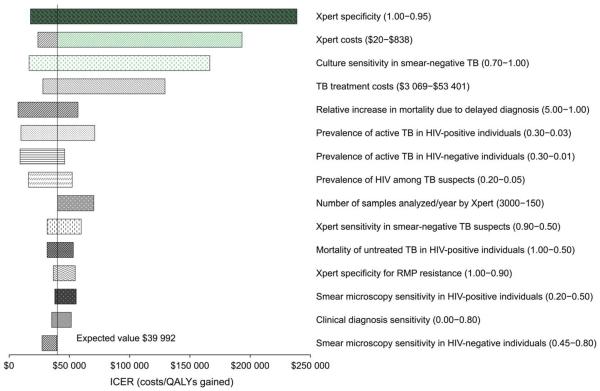
When comparing `intensive' Xpert implementation with `selective' Xpert implementation, the incremental cost-effectiveness ratio (ICER) was most influenced by the specificity of Xpert (range $18 630 to $230 471 per QALY-gained, for specificity from 100% to 95%), cost of Xpert (range $24 614 to $189 257 per QALY-gained for Xpert costs from $20 per test to $838 per test), and sensitivity of culture for smear-negative pulmonary TB ($16 440 per QALY-gained to $180 352 per QALY-gained for sensitivity range from 70% to 100%; Figure C.3).
Figure C.1.
One-way sensitivity analysis of incremental costs comparing `intensive' Xpert (Algorithm 5) vs. no molecular testing (Algorithm 1). Line represents incremental effects when using base-case estimates of all parameters. Not all parameters tested in sensitivity analysis are shown. TB = tuberculosis; HIV = human immunodeficiency virus; RMP = rifampin; MDR-TB = multidrugr esistant TB.
Figure C.2.
One-way sensitivity analysis of incremental QALYs gained comparing `intensive' Xpert (Algorithm 5) vs. no molecular testing (Algorithm 1). Line represents incremental effects when using base-case estimates of all parameters. Not all parameters tested in the sensitivity analysis are shown. TB = tuberculosis; HIV = human immunodeficiency virus; MDR-TB = multidrug-resistant TB; QALY = quality-adjusted life-years.
Figure C.3.
One-way sensitivity analysis of ICER comparing `intensive' Xpert (Algorithm 5) vs. `selective' Xpert (Algorithm 4). Line represents incremental effects when using base-case estimates of all parameters. Not all parameters tested in the sensitivity analysis are shown. All key variables were included in the sensitivity analysis. Only the top 15 variables influencing the ICER are shown; among the additional variables that were evaluated included the cost of MTD® testing, utility weights for TB disease and treatment, cost of TB and MDR-TB treatment, and probability of toxicity. TB = tuberculosis; HIV = human immunodeficiency virus; RMP = rifampin; ICER = incremental cost effectiveness ratio; QALY = quality-adjusted life year.
Figure C.4.
One-way sensitivity analysis of ICER comparing `intensive' Xpert (Algorithm 5) vs. `selective' MTD (Algorithm 2). Line represents incremental effects when using base-case estimates of all parameters. Not all parameters tested in the sensitivity analysis are shown. All key variables were included in sensitivity analysis. Only the top 15 variables influencing the ICER are shown; among the additional variables that were evaluated included cost of MTD testing, utility weights for TB disease and treatment, cost of TB and MDR-TB treatment, and probability of toxicity. TB = tuberculosis; HIV = human immunodeficiency virus; RMP = rifampin; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year.
APPENDIX D: PROBABILISTIC SENSITIVITY ANALYSIS RESULTS
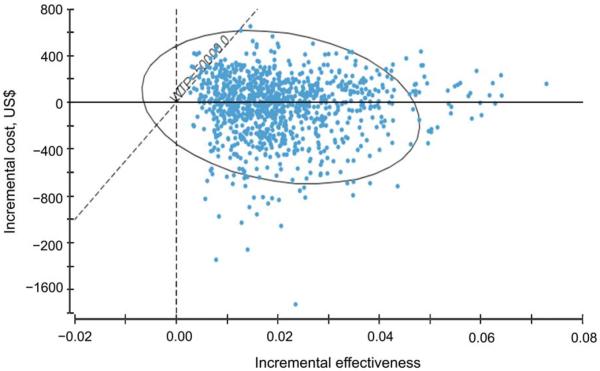
Probabilistic sensitivity analysis was conducted using Monte-Carlo simulation methods with 1000 iterations in which all key parameters are varied simultaneously (triangular, beta and log-normal distributions were used). Median costs, effects and ICERs are shown in Table D, along with a cost-effectiveness acceptability curve and scatterplot of costs and effects comparing `intensive' Xpert algorithm (Algorithm 5) with `no molecular testing' (Algorithm 1; Figure D). An algorithm using intensive testing of at least one sputum sample with Xpert (Algorithm 5) dominated the strategy of no molecular testing (Algorithm 1) in 53% of the simulations (i.e., was cost-saving), and was considered cost-effective at a willingness to pay (WTP) of $50 000 per QALY gained over 99% of the time. Compared to a strategy of `selective' molecular testing of only smear-positive samples with MTD, `selective' molecular testing with Xpert was considered highly cost-effective and was associated with a median ICER of $7972 per QALY gained (95%CI 6146–50 420). `Intensive' Xpert testing on at least one sputum sample for all TB suspects was associated with a median ICER of $16 165 per QALY-gained (95%CI 4886–84 896) compared to `selective' MTD testing. `Intensive' Xpert implementation was also considered cost-effective compared to `selective' Xpert implementation (median ICER $16 376 per QALY gained, 95%CI 4861–88 733).
Table D.
Probabilistic sensitivity analysis results
| Algorithm | Health system costs/TB suspect median (95%CI) |
Incremental costs/TB suspect median (95%CI)* |
QALYs accrued per patient median (95%CI) |
Incremental QALYs gained/ 1000 TB suspects (95%CI)* |
Median
ICER— cost/QALY gained (95%CI)* |
|---|---|---|---|---|---|
| 1: No molecular | 3172 (1728 to 6528) | Reference | 22.033 (21.95 to 22.089) | Reference | — |
| 2: Selective MTD | 2870 (1546 to 5688) | −288 (−1016 to −53) | 22.035 (21.95 to 22.090) | 1.5 (0 to 4.1) | Reference |
| 3: Intensive MTD | 3114 (1750 to 5946) | −35 (−837 to 332) | 22.050 (21.98 to 22.096) | 15.9 (5.0 to 41.0) | 17 487 (5 227 to 87 382) |
| 4: Selective Xpert | 2872 (1548 to 5697) | −284 (−1011 to −47) | 22.036 (21.95 to 22.090) | 2.0 (0.10 to 4.5) | 7972 (−6 146 to 50 420) |
| 5: Intensive Xpert | 3153 (1770 to 5987) | −11 (−824 to 375) | 22.053 (21.99 to 22.097) | 18.5 (5.6 to 47.1) | 16 165 (4 886 to 84 896) |
Incremental costs and effects and ICERs are calculated per simulation; median results with 95%Cls are reported. `Selective MTD' algorithm was used as Reference because the `No molecular' algorithm was dominated in the majority (53%) of simulations.
TB = tuberculosis; CI = confidence interval; QALY = quality-adjusted life year; ICER = incremental cost effectiveness ratio; MTD = Amplified Mycobacterium Tuberculosis Direct.
Figure D.
Incremental cost-effectiveness of Algorithm 5 vs. Algorithm 1 during iterations of Monte Carlo simulation. The ellipse represents 95% confidence points. Diagonal dashed line represents ICERs at a WTP threshold of $50 000. Points to the right of this dashed line are considered cost-effective. Dotted horizontal line shows incremental cost of $0. Points below this line represents i terations in which Algorithm 5 was cost-saving compared to Algorithm 1. WTP = willingness to pay; ICER = incremental cost-effectiveness ratio.
Footnotes
Conflict of interest: none declared.
The Appendices are available in the online version of this article at http://www.ingentaconnect.com/content/iuatld/ijtld/2013/00000017/00000010/art00014
References
- 1.Centers for Disease Control and Prevention . Reported tuberculosis in the United States—2011. CDC; Atlanta, GA, USA: 2012. [Accessed July 2013]. http://www.cdc.gov/tb/statistics/reports/2011/pdf/report 2011.pdf. [Google Scholar]
- 2.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 3.Maryland Department of Health & Mental Hygiene . Maryland TB guidelines for prevention and treatment of tuberculosis. DHMH; Baltimore, MD, USA: 2007. [Accessed July 2013]. http://ideha.dhmh.maryland.gov/OIDPCS/CTBCP/CTBCPDocuments/tbguidelines.pdf. [Google Scholar]
- 4.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. US Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health care facilities. Morb Mortal Wkly Rep. 2005;54(RR-17):1–141. [PubMed] [Google Scholar]
- 5.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–1227. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society . Rapid diagnostic tests for tuberculosis—workshop report. ATS; New York, NY, USA: 1997. [Accessed July 2013]. http://www.thoracic.org/statements/resources/mtpi/rapidtb1-12. pdf. [Google Scholar]
- 7.Centers for Disease Control and Prevention Update: nucleic acid amplification tests for tuberculosis. MMWR Morb Mortal Wkly Rep. 2000;49:593–594. [PubMed] [Google Scholar]
- 8.Dowdy DW, Maters A, Parrish N, Beyrer C, Dorman SE. Cost-effectiveness analysis of the Gen-Probe Amplified Myco-bacterium Tuberculosis Direct test as used routinely on smear-positive respiratory specimens. J Clin Microbiol. 2003;41:948–953. doi: 10.1128/JCM.41.3.948-953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and drug resistant tuberculosis: policy statement. WHO; Geneva, Switzerland: 2011. [Accessed July 2013]. WHO/HTM/TB/2011.4. http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. [PubMed] [Google Scholar]
- 12.Foundation for Innovative New Diagnostics . Negotiated prices for Xpert® MTB/RIF and FIND country list. FIND; Geneva, Switzerland: 2013. [Accessed July 2013]. http://www.finddiagnostics.org/about/what_we_do/successes/find-negotiated-prices/xpert_mtb_rif.html. [Google Scholar]
- 13.Cepheid . Pricing to the FIND target market of 145 countries. Cepheid; Sunnyvale, CA, USA: 2012. [Accessed July 2013]. http://www.cepheidcares.com/tb/cepheid-vision.html. [Google Scholar]
- 14.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high-burden countries: a cost-effectiveness analysis. PLoS Med. 2012;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown RE, Miller B, Taylor WR, et al. Health care expenditures for tuberculosis in the United States. Arch Intern Med. 1995;155:1595–1600. [PubMed] [Google Scholar]
- 16.de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med. 2009;169:179–187. doi: 10.1001/archinternmed.2008.524. [DOI] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality . Medical Expenditure Panel survey. AHRQ; Rockville, MD, USA: 2009. [Accessed July 2013]. http://meps.ahrq.gov/mepsweb/data_stats/summ_tables/hc/mean_expend/2009/table2.htm. [Google Scholar]
- 18.Kaiser Family Foundation . Hospital in-patient expenses. KFF; Menlo Park, CA, USA: 2012. [Accessed August 2012]. http://www.statehealthfacts.org/comparemaptable.jsp?ind=273&cat=5. [Google Scholar]
- 19.Rajbhandary SS, Marks SM, Bock NN. Costs of patients hospitalized for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:1012–1016. [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman D, Schlaeffer F, Boldur I, et al. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax. 1996;51:179–184. doi: 10.1136/thx.51.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rimland D, Navin TR, Lennox JL, et al. Prospective study of etiologic agents of community-acquired pneumonia in patients with HIV infection. AIDS. 2002;16:85–95. doi: 10.1097/00002030-200201040-00011. [DOI] [PubMed] [Google Scholar]
- 22.Colice GL, Morley MA, Asche C, Birnbaum HG. Treatment costs of community-acquired pneumonia in an employed population. Chest. 2004;125:2140–2145. doi: 10.1378/chest.125.6.2140. [DOI] [PubMed] [Google Scholar]
- 23.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention/American Thoracic Society Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 26.Steingart KR, Ng V, Henry M, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:664–674. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 27.Guerra RL, Hooper NM, Baker JF, et al. Use of the Amplified Mycobacterium Tuberculosis Direct test in a public health laboratory: test performance and impact on clinical care. Chest. 2007;132:946–951. doi: 10.1378/chest.06-2959. [DOI] [PubMed] [Google Scholar]
- 28.Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitzgerald JM. Health state utilities in latent and active tuberculosis. Value Health. 2008;11:1154–1161. doi: 10.1111/j.1524-4733.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 29.Sohn H, Minion J, Albert H, Dheda K, Pai M. TB diagnostic tests: how do we figure out their costs? Expert Rev Anti Infect Ther. 2009;7:723–733. doi: 10.1586/eri.09.52. [DOI] [PubMed] [Google Scholar]
- 30.Shah M, Miele K, Choi H, et al. QuantiFERON-TB Gold In-Tube implementation for latent tuberculosis diagnosis in a public health clinic: a cost-effectiveness analysis. BMC Infect Dis. 2012;12:360. doi: 10.1186/1471-2334-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 32.Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716–717. doi: 10.1046/j.1525-1497.1998.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization . Implementation and roll-out of Xpert MTB/RIF. WHO; Geneva, Switzerland: 2012. [Accessed July 2013]. May 2012 update. http://www.stoptb.org/wg/gli/assets/documents/ Xpert%20MTB-RIF%20UPDATE%20May%202012.pdf. [Google Scholar]
- 34.Schnippel K, Meyer-Rath G, Long L, et al. Scaling up Xpert MTB/RIF technology: the costs of laboratory- vs. clinic-based roll-out in South Africa. Trop Med Int Health. 2012;17:1142–1151. doi: 10.1111/j.1365-3156.2012.03028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS ONE. 2012;7:e36966. doi: 10.1371/journal.pone.0036966. [DOI] [PMC free article] [PubMed] [Google Scholar]