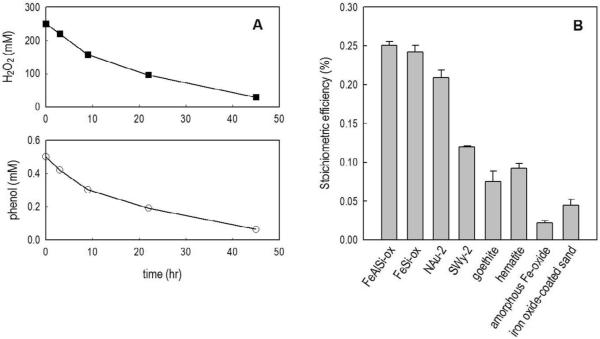
Figure 1.
(A): A representative example of H2O2 decomposition and phenol transformation. [NAu-2] = 5 g/L, pH = 8.4 ± 0.1, [borate] = 2 mM. (B): Stoichiometric efficiency (Δ[phenol]/Δ[H2O2] × 100%) with different iron-containing minerals. [H2O2]0 = 250 mM, [phenol]0 = 0.5 mM, [FeSi-ox] = [FeAlSi-ox] = 10 g/L, [NAu-2] = 5 g/L, [Swy-2] = 10 g/L, [hematite] = [goethite] = 10 g/L, [FeOOH] = 1 g/L, [Fe-coated sand] = 10 g/L, pH = 8.4 ± 0.1.