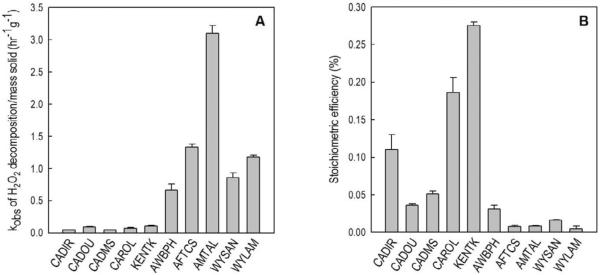
Figure 2.
Mass-normalized observed-first order rate constants for H2O2 decomposition (A) and stoichiometric efficiency (B) with aquifer materials. [H2O2]0 = 250 mM, [phenol]0 = 0.5 mM, [solid] was either 150 g/L (for CADIR, CADOU and CADMS) or 50 g/L (for all other aquifer materials), [borate] = 10 mM, pH = 8.2 – 8.7, except in experiments with KENTK, in which the pH was 7.2 (see Table 2). The observed-first order rate constants (kobs) were obtained by fitting the experimental data to the first order decay reaction rate law. The r2 values of the fittings were always r2 > 0.99.