Abstract
Osteoporosis is a skeletal system pathology characterized by low bone mineral density and tissue structural deterioration. This malady is associated with high fracture risk that severely compromises quality of life. Osteoporosis incidence is becoming more significant with increasing lifespan worldwide. However, current approaches for treating osteoporosis cannot and do not treat the disease in the most ideal manner for diverse reasons. Substantial research has sought both the discovery of new targets and new therapies. In this review, emerging possible RNAi-mediated therapeutic opportunities for osteoporosis are identified and associated challenges discussed. Targeted delivery strategies capable of more reliable and efficient delivery to skeletal tissue are described, as well as possibilities to treat bone-forming cells with siRNA to produce cell-based therapy.
Current therapeutic strategies for osteoporosis
Normal bone maintains healthy bone modeling and remodeling dynamics under the control of endogenous signals that regulate continuous cell-based bone deposition and resorption. Bone modeling involves osteoblasts that continuously deposit new bone without prior bone resorption – a dominant activity during early bone growth to peak body and skeletal mass. Bone remodeling involves new bone deposited by osteoblasts at bone resorption pits previously generated by osteoclasts where new bone formation by osteoblasts is tightly balanced with old bone resorption. Balanced bone remodeling is a hallmark of bone homeostasis in adults where bone turnover occurs continuously.
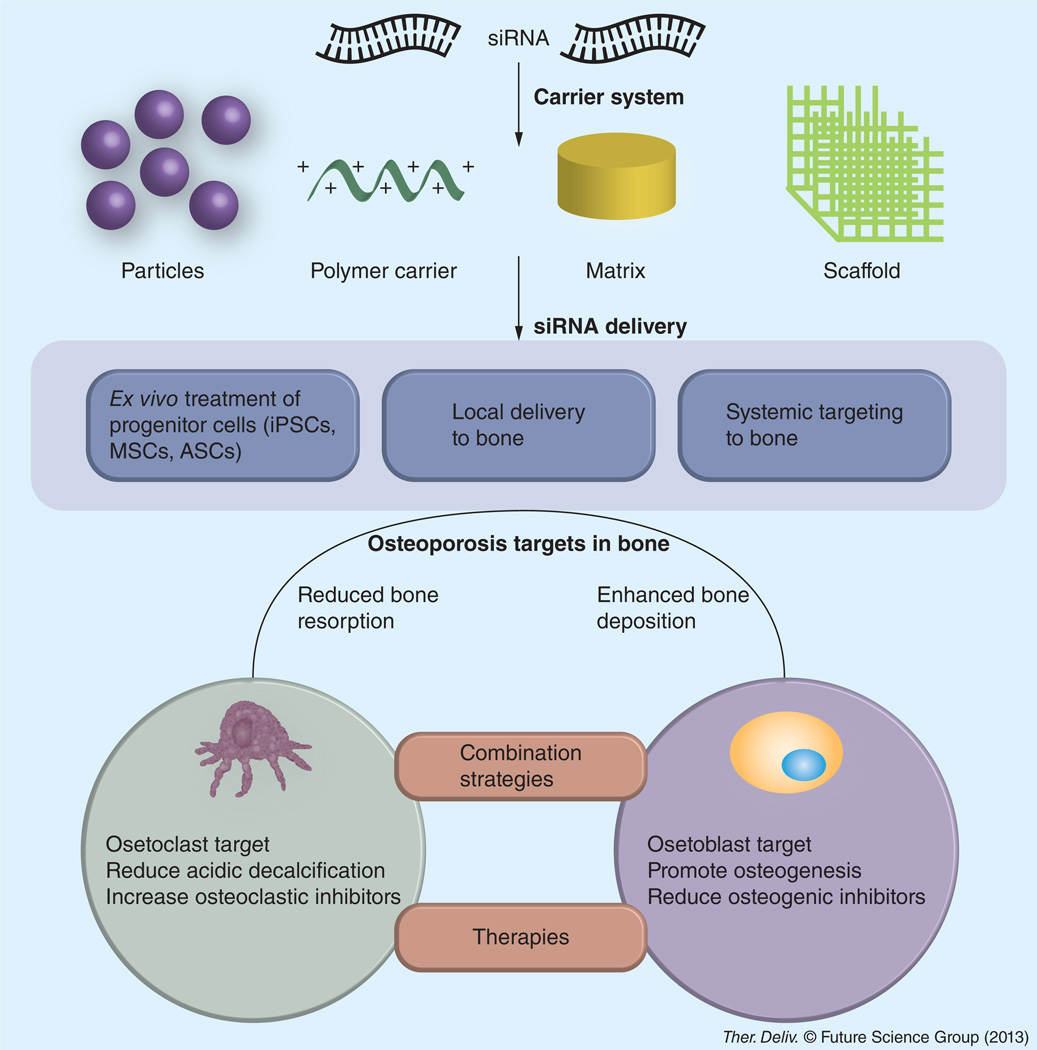
Osteoporosis is a systemic disease characterized by bone formation rates that are much slower than bone resorption rates, resulting in net bone loss and increased risk of fracture from low bone mineral density and quality. Therefore, current therapeutic strategies for osteoporosis either seek to inhibit bone resorption using administered antiresorptive agents or to enhance bone formation using anabolic drugs. Clinically available antiresorptive agents include bisphosphonates, estrogens, selective estrogen receptor modulators, calcium and vitamin D and the newly introduced protein drug, denosumab. Parathyroid hormone (PTH) is the only anabolic treatment so far in clinical use. Long-term administration of antiresorptive drugs is reported to impair bone formation, for example, inducing jaw osteonecrosis and atypical fractures in long bones with bisphosphonate use [1,2]. PTH promotes osteoblast differentiation while inhibiting apoptosis [3]. However, PTH also promotes osteoclastogenesis and natural bone resorption activity so that chronic exposure to high, continuous serum PTH levels apparently nullifies its anabolic action in promoting bone formation and results in bone resorption [4,5]. Methods to control PTH pharmacodynamics to maintain consistent anabolic bone formation activity in vivo are required to elicit an appropriate treatment for osteoporosis. Hence, while clinical therapeutics are offered to combat increasing osteoporosis incidence observed worldwide, none are without adverse effects. Analysis of current treatments for osteoporosis produces the conclusion that desirable new drugs should either prevent bone resorption but not impair bone formation, or promote bone deposition without altering bone resorption, or both in careful synergy. This drug-development concept is shown in Figure 1. Development of improved therapeutic agents is warranted, especially those that reliably produce dual efficacy in limiting bone resorption while promoting safe, therapeutic osteogenesis.
Figure 1. Design concept for RNAi-based therapeutics targeting osteoporosis.
Carrier-mediated siRNA delivery, either locally or systemically, is directed to inhibit specific osteoclast signals involved with bone resorption or to augment osteoblast signals that elicit osteogenesis, or both simultaneously in combination therapies (shown at base of figure). Strategies can be used ex vivo in pretreating bone-forming cells to be implanted as cell-based therapies into bone, or in vivo by directed, targeted siRNA delivery to disease sites in the musculoskeletal system.
ASC: Adipocyte-derived stem/stromal progenitor cell; iPSC: Induced pluripotent stem cell; MSC: Mesenchymal stromal cell.
siRNA therapeutics
RNAi is a natural process used by living cells to silence specific gene expression through a sequence-directed highly precise and transient mechanism focused at messenger RNA in the cell cytoplasm. The process does not directly involve DNA and therefore bypasses nuclear processes and complex transport through the nuclear envelope. Theoretically, RNAi mechanisms can be exploited for therapeutic purposes to down-regulate any disease-related pathogenic gene of interest by deliberately feeding the specific RNAi pathway for that targeted gene with small pieces of RNA that bind mRNA produced from that gene. This phenomenon is currently exploited as a tool to study specific gene knockdown in cell signaling. siRNA is a subset of RNAi-based approaches increasingly investigated for therapeutic purposes using exogenous delivery of carefully designed short RNA sequences complimentary to disease-causing mRNA transcripts produced in diseased cells [6]. Some siRNA agents are currently in clinical development [7]. Generally, RNAi methods represent a very powerful tool to elucidate gene function, but their translation to therapeutics has struggled to overcome a major hurdle in therapeutics: their efficient, reliable delivery to the disease site. Naked siRNA molecules injected into the blood stream or tissues are vulnerable to degradation and off-site bioactivities, and are unable to effectively penetrate cell membranes alone because of their relatively large molecular size and high anionic charge density. Therefore, for siRNA therapeutics to become more effective and attractive as a versatile drug form, improved delivery systems are required in vivo. In the case of osteoporosis treatments, siRNA therapeutics require a reliable mRNA disease-specific target in bone remodeling, a method to deliver siRNA to that target within the bone cell cytoplasm at reasonable doses (potency) sufficient to produce siRNA effects, disease specificity to avoid gene knockdown at nondisease sites, and a therapeutic index wide enough to accommodate some dosing variability that avoids toxicity while eliciting therapeutic effects.
siRNA targets for osteoporosis therapeutics
siRNA sequences targeting gene transcripts that only negatively regulate bone formation without modulating bone resorption, or those that only stimulate bone resorption without modulating bone formation could actually increase bone mass. In addition, targeting gene knockdown specific to skeletal tissues would avoid undesired knockdown in nondisease organs, removing common causes of adverse effects. A precise, specific siRNA target also compensates for poor targeting ability from the delivery system, enhancing both safety and efficacy in many cases. Bone metabolic regulatory pathways have been a focus for more than a decade. This has enhanced the collective understanding of cellular and molecular events in bone biology relevant to osteoporosis, for example, the elucidation of the Wnt/β-catenin (osteogenesis) and RANKL/RANK pathways (osteoclastogenesis) [8]. Genes vital to these signal transduction mechanisms have been identified and serve as potential siRNA target candidates for knockdown. A summary of all reported targets for inhibiting osteoclast-mediated bone resorption was published recently [9]. More attention is now being paid to the role of osteoblasts in osteoporosis. Signaling molecules that are positive regulators of osteoblasts and that stimulate osteogenesis to increase bone mass are often controlled by inhibitory partners. Knockdown of targets that inhibit osteoblast formation, their differentiation, maturation and activation, or that induce osteoblast apoptosis should also lead to increased bone mass. This could be achieved by knockdown of these negative signals. For example, proteins sclerostin and DKK1 are both endogenous negative regulators of bone formation by inhibiting Wnt signaling [10,11]. Antibodies against sclerostin are currently in Phase III clinical trials for osteoporosis therapy, and antibodies against DKK1 are being tested for preventing osteolytic bone disease in multiple myeloma patients. The action of antibody blockade of these bone-regulating targets could be duplicated using siRNA knockdown of sclerostin or DKK1. Since the first aptamer-based drug, pegaptanib (Macugen™, OSI Pharmaceuticals) was US FDA-approved for age-related macular degeneration, other DNA aptamers have also been investigated as new bone-forming agents. Shum et al. identified a DNA aptamer with high affinity for sclerostin. Inhibition of sclerostin-mediated antagonism of Wnt signaling by the aptamer in osteoblast MC3T3-E1 cell culture was dose-dependent. In addition to utility as therapeutics, aptamers can be utilized as targeting motifs as well. A study combining aptamers with therapeutic siRNA to target tumor tissue has been reported [12]. Therefore, aptamers could be used for bone cell-specific delivery. Other negative bone mass regulators reported recently include osteoblast-specific Y1 [13], PTK2 [14], PPAR-γ [15], and zinc finger protein 467 that inhibits adipose-derived stem cell differentiation to osteoblasts [16]. Again, siRNA targeting of these ‘negative’ signaling proteins that hinder bone production could yield therapeutically interesting bone metabolic changes, prompting some attention to these as possible pharmacological candidates for osteoporosis. Nonetheless, as with most medications, side effects are possible: therapeutics acting on the Wnt pathway are known to induce cancer by continuously stimulating Wnt activation [17].
Lastly, targets are also reported that both positively influence osteoblasts and negatively signal osteoclasts simultaneously, such as FPPS and nuclear factor of active T cells (NFATc1) [15,18]. FPPS is the major intracellular target of the drug alendronate, one of the most used pharmacological treatments for osteoporosis. FPPS is a key enzyme in the mevalonate pathway which produces lipids that are critical for post-translational prenylation of proteins regulating cell apoptosis [19]. By inhibiting FPPS, post-translational prenylation of GTP-binding proteins is down-regulated, inducing apoptosis of osteoclasts [20]. Interestingly, inhibiting the mevalonate pathway also showed induced osteoblast differentiation and mineralization [21]. Consistently, siRNA targeting FPPS significantly suppressed osteoclast viability and increased osteoblast differentiation [22]. siRNA targeting NFATc1 was also shown to inhibit LPS-induced osteoclast formation in murine RAW264.7 cells [23]. In addition, significant reduced RANKL stimulation of MMP-9 activity during osteoclast differentiation was observed after siRNA silencing NFATc1 in RAW164.7 cells [24]. However, NFATc1 was shown to negatively regulate osteoblast differentiation in MC3T3-E1 preosteoblast cells, and its silencing with siRNA increased Fra-2 expression [25]. Specifically inhibiting these cell modulators can therefore inhibit osteoclast-mediated bone resorption while promoting osteoblast-mediated bone resorption. These targets therefore represent novel candidates for the development of anabolic treatments for osteoporosis. Nonetheless, most of these effects have been shown in simplified cell culture models where siRNA is dosed directly to the media. Delivery of therapeutically relevant doses of siRNA to in vivo models has proven much more challenging [26,27], so identification of targets is only one part of a complex development process for a new siRNA-based therapeutic for osteoporosis.
Osteoporosis patients have a very high fracture risk, occurring primarily at the hip, spine and wrist. Many treatments involve orthopedic surgeries. Bone healing processes under osteoporotic conditions are difficult, and become more complicated in the presence of fixation hardware. To enhance and improve healing, local delivery of bone growth factors that promote bone growth, or antiresorptive therapeutics, have been investigated as supplements in combination with surgery. Rapidly developing cell therapeutics and bone tissue engineering strategies offer another potential solution should they become off-the-shelf, reliable bone-healing technologies. Induced pluripotent stem cells (iPSCs), mesenchymal stromal cells (MSCs) and adipocyte-derived stem/stromal progenitor cells (ASCs) all have the potential to differentiate into a variety of cell types, including bone cells. Importantly, they can be readily sourced or derived from both allogenic and autologous tissues in large quantities. The recognized immune-modulatory properties of ASCs and MSCs [28,29] is beneficial for their possible generic cross-patient use as products for bone therapies. One major therapeutic challenge for these cells is tight control over cell differentiation and differentiated stability when placed into human tissue sites. Cell differentiation into specific lineages can be regulated by culture under defined conditions using growth factors that control specific pathways. Alternatively, siRNA can also be used, both ex vivo and in vivo, to regulate progenitor cell differentiation pathways involved in osteogenic cell production for bone regeneration [30]. Down-regulating cell inhibitory gene signals using RNAi-based mechanisms to directly promote osteogenic differentiation can facilitate bone formation. For example, the transcriptional regulator, core-binding factor α-1 is strictly specific to osteoblasts, playing an essential role in regulating osteogenic differentiation [31]. Using siRNA to knock down its negative regulator, GNAS1, results in increased expression of core-binding factor α-1 and production of bone-differentiating proteins, such as type I collagen, osteocalcin and osteopontin [32]. Many other cell pathways are potential targets for siRNA-based regulation of bone homeostasis; cell-based therapies in osteoporosis could find benefit from cellular control mechanisms offered by siRNA knockdown of select positive and negative regulators involved in bone turnover.
Bone tissue engineering strategies in osteoporotic fracture exploits the capabilities to deliver differentiated osteogenic cells, or their progenitor cells (e.g., iPSCs, ASCs, MSCs), as well as siRNA for guiding these cells towards osteogenic potential, all within the implanted scaffolding materials. A study evaluating cotransfecting human adipose-derived stem cells with osteoinductive DNA (BMP2) and siRNA against BMP antagonists, Noggin and GNAS, was reported [33]. Co-delivery of these agents significantly accelerated stem cell osteoblastic differentiation, demonstrated by significant increases in the expression of bone markers and mineralization of human adipose-derived stem cells. These results suggest that siRNA can be synergistically used to accelerate stem cell differentiation to osteogenic potential, and possibly used together in cell-based delivery materials in cell therapies for bone. A nanoparticle-mediated siRNA delivery system within a scaffold implant was designed to knock down both BCL2L2 to enhance osteogenic differentiation, and TRIB2 to promote adipogenic differentiation. TransIT-TKO®-siRNA nanoparticles were coated onto surfaces of biodegradable polycaprolactone scaffolds to deliver the carrier to attaching cells. Successful knockdown was obtained in human MSCs cultured in vitro. For in vivo evaluation, these scaffolds were seeded with hMSCs for 16 h before subcutaneous implantation in a mouse model, and harvested 8 weeks post-implantation; enhanced adipogenic and osteogenic cell differentiation were observed [34]. A very interesting dual differentiation was shown in scaffolds both in vitro and in vivo when one side of the scaffold cylinder was coated with BCL2L2 siRNA and the other side was coated with TRIB2 siRNA. Stem cells were shown to be directed into different differentiation pathways within one implant due to different siRNAs localized to spatially discrete locations [34]. A recent study using siRNA against GNAS1 and PHD2 in MSCs tested the effects of simultaneously dosing these two siRNA on bone formation in a sheep model. siRNA molecules were embedded within silk fibroin–chitosan biomaterials placed in plastic chambers sutured ectopically onto grafted periosteum on the latissimusdorsi muscle in sheep. The in vivo data showed increased bone volume 70 days post-implantation in animals treated with each siRNA alone or siRNAs targeting both genes. Consistent bone density was obtained in the newly forming bone in the silk fibroin–chitosan scaffolds during 70 days post-implantation.
siRNA delivery to bone
A primary hindrance to siRNA therapeutics development across many therapeutic opportunities is the inability to reliably deliver siRNA molecules to target organs or cells. Investigating topical siRNA therapies have been particularly attractive for skin diseases because direct application to disease sites allows more effective siRNA delivery [35]. Beyond topical delivery, strategies to systemically deliver siRNA in vivo to date use both nonviral and viral carriers for delivery, currently emphasizing nonviral carriers given long-standing safety and efficacy concerns with viral transfection agents [6]. Although viral delivery shows much longer duration and potentially improved patient compliance, these carriers are always accompanied by intrinsic pathogenic, immunologic and safety concerns, limiting their consideration in therapeutic applications for nonlethal diseases such as osteoporosis [36]. Systemic administration of siRNA might be preferable as osteoporosis is a systematic disease. In addition, there is a clear shift in siRNA therapeutics under clinical development from local to systemic delivery in order to use well-established systemic delivery platforms with improved stability for nucleic acid payloads [7]. Compared with investigations that apply siRNA therapeutics to soft tissue diseases, such as liver, kidney and lung, studies delivering siRNA to bone for osteoporosis are much less developed.
Systemic targeted-delivery strategies typically use known bone-homing chemistry to bind mineralized phases of inorganic bone. For example, one recent approach describes a promising new targeting system for specifically delivering siRNA to bone-forming surfaces using a novel siRNA delivery system comprising a carrier currently FDA approved for clinical trials – dioleoyltrimethylammonium propane-based liposomes conjugated with a targeting peptide (AspSerSer)6 [37]. Bone targeting with this peptide motif is based on peptide recognition of different degrees of crystallized hydroxyapatite on new bone surfaces. Bone-forming surfaces have highly crystallized hydroxyapatitie compared with low crystallized hydroxyapatitie on bone resorption surfaces [38]. Based on the known higher affinity of (AspSerSer)6 for small, randomly oriented crystals [39], the authors tested its ability to target bone-forming surfaces by tail vein injection of (AspSerSer)6-FITC into rodents followed by subcutaneous injection of xylenol orange specifically labeling bone-forming surfaces. Confocal laser scanning microscopy of bone sections showed significant dual fluorescence signal overlap. They chose Plekhol, a negative regulator of osteoblasts, as their siRNA target. This system showed both bone and osteogenic cell-selective delivery and knockdown efficiency in OVX rats. RNAi-mediated anabolic action was examined in both healthy and OVX rats by tail vein injection and both models showed significant increases in bone mineral density and largely improved three-dimensional architecture parameters in trabecular bone of the proximal tibia. Especially in OVX groups, all micro-computed tomography parameters for bone in the treated group recovered nearly to presurgery values after 9-week intravenous injections of carrier-siRNA at intervals of 1 week (dosing 3.75 mg/kg siRNA).
For in vivo local bone induction, it is not necessary to directly target genes regulating bone remodeling. A local delivery system utilizing a biodegradable hydrogel carrier for siRNA designed to enhance BMP-induced bone showed greater bone formation compared with BMP alone in mice [40]. The hydrogel had previously been developed for in vivo delivery of the osteogenic protein therapy, BMP-2. The siRNA inhibited noggin, a major antagonist to BMPs. Enhanced endogenous BMP-2 bioavailability induced new bone formation as confirmed by x-ray radiography and bone densitometry. This strategy combining both BMP-2 and Noggin siRNA produces greater ectopic bone formation than BMP-2 alone by using siRNA to stimulate BMP bioactivity by blocking its inhibitor. Chordin is another inhibitor of BMPs; its knockdown showed significantly increased expression of alkaline phosphatase as an osteogenic marker and increased extracellular mineral deposition in human MSC culture in osteogenic differentiation media [41]. This broadens the application of siRNA designs in possible osteoporosis treatment strategies by targeting both endogenous positive regulators of bone formation by knocking down their antagonists.
A local delivery system targeting osteoclast-mediated excessive bone resorption was also reported recently [42]. The intent was to locally inhibit osteoclast activity to facilitate implant stabilization, integration and healing under osteoporotic conditions. Injectable calcium phosphate cement (CPC) used routinely in orthopedic surgery was used as the in situ depot-forming siRNA delivery reservoir. Osteoclasts and their precursor cells were targeted as professional phagocytes in bone. siRNAs were complexed with polycations and encapsulated in degradable polymer polylactic-co-glycolic acid particles of micron sizes capable of cell uptake exclusively by phagocytosis. Particles were size-controlled in micron-size ranges in order to be taken up only by osteoclasts and their phagocytic precursors at the implantation site. These siRNA-containing particles were dispersed throughout CPC prior to its setting in situ, to be released upon degradation of CPC in vivo. The system was tested using in vitro culture and showed successful prolonged knockdown effects in osteoclasts. CPC has already been investigated as a bone drug carrier for conventional small molecule and protein-based drugs, and also could be utilized as promising local delivery matrix for siRNA drugs [9].
Figure 1 summarizes the approaches described here for delivering siRNA therapeutics to bone to address osteoporosis. RNAi delivery ex vivo to progenitor cells capable of osteogenesis can be used to stimulate and direct their osteogenic potential. These treated cells can then be implanted for therapeutic use. In vivo delivery of siRNA can exploit either local delivery from bone implants or systemic delivery that must have targeting specificity to bone sites, more specifically sites of osteoporosis pathogenesis. In all of these cases, appropriate RNAi targets would be those that either knockdown pathways promoting osteoclastogenesis or bone resorptive capacities of osteoclastic cells, or those that promote osteogenesis pathways in osteoblastic cells by knocking out pathways inhibiting osteogenesis.
Future perspective
With the discovery of key regulatory genes for osteogenesis and osteoclastogenesis, identifying therapeutic targets no longer seems to be a challenge for siRNA drug development. ‘Multitargeting’ designs have been exploited for siRNA therapeutics for treating complex diseases by addressing several targets at once. This idea could be a future direction for treating osteoporosis using siRNAs to direct cell behaviors, both in ex vivo culture prior to cell implantation in cell-based therapeutics (i.e., using iPSCs, MSCs or ASCs), or in vivo with siRNA alone, more reliably and efficiently targeted to osteoporotic sites than currently possible. In addition, the strategy allows the directing of siRNA combination therapies to both positively regulate osteoblasts and negatively regulate osteoclasts simultaneously. The big hurdle for the development and optimization of RNAi-mediated therapies is the lack of efficient delivery systems to target specific cells and to provide reliable dosing for signal modulation and target knockdown at disease sites without off-target adverse effects. This essential therapeutic requirement (i.e., safety, specificity, efficacy) should be the focus of next development steps for siRNA therapeutics in osteoporosis. Existing carrier and delivery technologies now known for gene therapy (see concepts presented in Figure 1, e.g., polyplexes, colloids, particles, viral and viral-like carriers) can also be modified to address siRNA delivery needs, given the many physical, chemical and disease-targeting similarities between siRNA and DNA delivery strategies. This will address siRNA delivery challenges more directly and rapidly to potentially facilitate needed improvements for improved delivery to bone to treat osteoporosis. To further develop these carrier systems specially targeting osteoporotic bone, a highly effective targeting mechanism needs to be incorporated to direct siRNA dosing to the diseased site without significant off-target issues. In addition, the targeted therapy needs to be directed specifically to osteoclasts and osteoblasts involved in bone turnover to produce a new homeostasis for normal bone metabolism and turn-over. Should meeting these challenges be successful, RNAi-mediated therapies will become a more interesting and attractive clinical candidates, where reduced risk and enhanced efficacy impacts their utility in future approaches for osteoporosis treatment.
Executive summary.
The need for new osteoporosis therapies
-
▪
Current drugs provide modest therapeutic benefit (bone re-mineralization) with significant risks for undesired and even dangerous side effects (e.g., osteonecrosis, bone healing impairment).
-
▪
Most current treatments focus on inhibiting bone resorption, but can actually also suppress bone remodeling rates to end up decreasing new bone formation rates.
-
▪
Osteoporosis is increasing with an aging international population, including both males and females.
The value of targeting siRNA to osteoporotic bone
-
▪
siRNA can knockdown very specific bone metabolic and catabolic pathways, and its bioactivity can be reversed with time if required.
-
▪
siRNA can be designed to target signaling molecules that are negative regulators of osteoblast-mediated bone formation to increase bone mass as new bone anabolic therapy.
-
▪
siRNA can be used adjunct to clinically common drug therapies used currently.
-
▪
Different siRNAs can be used simultaneously in therapy to enhance bone metabolic and block catabolic pathways known to be imbalanced in osteoporosis.
-
▪
siRNA can be packaged within known drug carriers to modify its delivery, biodistribution and other important therapeutic properties within host tissue.
-
▪
Osteoclasts are professional phagocytes and can be targeted with particle carriers that will not readily enter most other cells, allowing delivery specificity to osteoclasts.
-
▪
Several targeting agents (peptides, bisphosphonates) are known to bind selectively to bone mineral phases.
-
▪
Bone augmentation methods to enhance osteoporotic bone mechanics using biomaterials in bone could also serve as drug delivery devices.
Challenges in siRNA therapeutics for osteoporosis
-
▪
Targeted systemic delivery of therapeutic amounts of siRNA continues to prove difficult.
-
▪
siRNA off-target effects can be worrisome.
Acknowledgments
The authors declare partial research financial support from internal University of Utah research funds (SEED program, and George S and Dolores Doré Eccles Presidential Chair Endowment) and from NIH (USA) grant EB-00894.
Key Terms
- Osteoporosis
Bone disease characterized by reduced bone mineral density, deterioration of bone micro-architecture and increased risk of fracture.
- Anabolic drugs
Therapeutics that enhance osteoblast formation, maturation and deposition of new bone.
- Antiresorptive drugs
Therapeutics that inhibit osteoclast formation, activation and survival to limit bone removal.
- Osteonecrosis
Bone tissue death caused by bone cell death from a diverse array of different stresses in vivo.
- siRNA therapeutics
Using siRNA as active pharmaceutical ingredient to down-regulate disease target expression and produce therapy.
- siRNA delivery
Due to siRNA’s negative charge, immune activation potential and rapid degradation in vivo, effective delivery systems must be designed to protect siRNA from degradation and deliver these molecules to the intended disease sites at proper dosing and timing regimens for therapy.
- Bone targeting
Effective and reliabe deposition of drugs in bone tissues at the corrrect dose to yield therapeutic value.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- 1.Hollick RJ, Reid DM. Role of bisphosphonates in the management of postmenopausal osteoporosis: an update on recent safety anxieties. Menopause Int. 2011;17(2):66–72. doi: 10.1258/mi.2011.011014. [DOI] [PubMed] [Google Scholar]
- 2.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N. Engl. J. Med. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 3.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Nishida S, Boudignon BM, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J. Bone Miner. Res. 2007;22(9):1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle DD, Sakata T, Leary C, et al. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J. Bone Miner. Res. 2002;17(9):1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 6. Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007;59(2–3):75–86. doi: 10.1016/j.addr.2007.03.005. ▪ Provides a detailed introduction to the mechanism of RNAi and discusses its potential problems and strategies for therapeutic applications.
- 7. Haussecker D. The business of RNAi therapeutics in 2012. Mol. Ther. Nucleic Acids. 2012;1:e8. doi: 10.1038/mtna.2011.9. ▪ Discusses the development, current status, prospects and opportunities for RNAi therapeutics from the perspective of pharmaceutical products and commercialization.
- 8.Gogakos AI, Bassett JHD, Williams GR. Bone signaling pathways and treatment of osteoporosis. Expert Rev. Endocrinol. Metab. 2009;4(6):639–650. doi: 10.1586/eem.09.38. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Grainger DW. RNA therapeutics targeting osteoclast-mediated excessive bone resorption. Adv. Drug Deliv. Rev. 2012;64(12):1341–1357. doi: 10.1016/j.addr.2011.09.002. ▪ Summarizes the reported cellular signaling targets that play important roles in osteoclast differentiation, activation and function, and which could potentially serve as siRNA targets to inhibit osteoclast-mediated excessive bone resorption. Specific siRNA bone delivery is also discussed in this review.
- 10.Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J. Bone Miner. Res. 2009;24(4):578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 11.Morvan F, Boulukos K, Clement-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 12.Mcnamara JO, 2nd, Andrechek ER, Wang Y, et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006;24(8):1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 13.Lee NJ, Nguyen AD, Enriquez RF, et al. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011;48(3):461–467. doi: 10.1016/j.bone.2010.10.174. [DOI] [PubMed] [Google Scholar]
- 14.Buckbinder L, Crawford DT, Qi H, et al. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc. Natl Acad. Sci. USA. 2007;104(25):10619–10624. doi: 10.1073/pnas.0701421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Q, Zhang XL, Dai KR, Benderdour M, Fernandes JC. siRNA therapy for cancer and non-lethal diseases such as arthritis and osteoporosis. Expert Opin. Biol. Ther. 2011;11(1):5–16. doi: 10.1517/14712598.2010.532483. [DOI] [PubMed] [Google Scholar]
- 16.You L, Pan L, Chen L, et al. Suppression of zinc finger protein 467 alleviates osteoporosis through promoting differentiation of adipose derived stem cells to osteoblasts. J. Transl. Med. 2012;10:11. doi: 10.1186/1479-5876-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev. Dyn. 2010;239(1):102–114. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo H, Mcdonald JM, Zayzafoon M. NFATc1: a novel anabolic therapeutic target for osteoporosis. Ann. NY Acad. Sci. 2006;1068:564–567. doi: 10.1196/annals.1346.053. [DOI] [PubMed] [Google Scholar]
- 19.Lacal JC. Regulation of proliferation and apoptosis by Ras and Rho GTPases through specific phospholipid-dependent signaling. FEBS Lett. 1997;410(1):73–77. doi: 10.1016/s0014-5793(97)00444-4. [DOI] [PubMed] [Google Scholar]
- 20.Tenenbaum HC, Shelemay A, Girard B, Zohar R, Fritz PC. Bisphosphonates and periodontics: potential applications for regulation of bone mass in the periodontium and other therapeutic/diagnostic uses. J. Periodontol. 2002;73(7):813–822. doi: 10.1902/jop.2002.73.7.813. [DOI] [PubMed] [Google Scholar]
- 21.Reinholz GG, Getz B, Sanders ES, et al. Distinct mechanisms of bisphosphonate action between osteoblasts and breast cancer cells: identity of a potent new bisphosphonate analogue. Breast Cancer Res. Treat. 2002;71(3):257–268. doi: 10.1023/a:1014418017382. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Panasiuk A, Grainger DW. Small interfering RNA knocks down the molecular target of alendronate, farnesyl pyrophosphate synthase, in osteoclast and osteoblast cultures. Mol. Pharm. 2011;8(4):1016–1024. doi: 10.1021/mp100374n. ▪ Describes use of siRNA to target FPPS, the cellular target of bisphosphonates – a clinical, widely used treatment for postmenopausal osteoporosis. The strategy seeks to use siRNA to inhibit osteoclast bone resorption but avoid common side effects from drugs like bisphosphonates that also target FPPS. The paper provides a new therapeutic direction for siRNA therapeutics.
- 23.Fahid FS, Jiang J, Zhu Q, et al. Application of small interfering RNA for inhibition of lipopolysaccharide-induced osteoclast formation and cytokine stimulation. J. Endod. 2008;34(5):563–569. doi: 10.1016/j.joen.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Sundaram K, Nishimura R, Senn J, Youssef RF, London SD, Reddy SV. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007;313(1):168–178. doi: 10.1016/j.yexcr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Yeo H, Beck LH, Mcdonald JM, Zayzafoon M. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone. 2007;40(6):1502–1516. doi: 10.1016/j.bone.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiemann K, Rossi JJ. RNAi-based therapeutics-current status, challenges and prospects. EMBO Mol. Med. 2009;1(3):142–151. doi: 10.1002/emmm.200900023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gary DJ, Puri N, Won YY. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J. Control. Release. 2007;121(1–2):64–73. doi: 10.1016/j.jconrel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449–1470. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 29.Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33(32):8142–8151. doi: 10.1016/j.biomaterials.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benoit DS, Boutin ME. Controlling mesenchymal stem cell gene expression using polymer-mediated delivery of siRNA. Biomacromolecules. 2012;13(11):3841–3849. doi: 10.1021/bm301294n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 32.Rios CN, Skoracki RJ, Mathur AB. GNAS1 and PHD2 short-interfering RNA support bone regeneration in vitro and in an in vivo sheep model. Clin. Orthop. Relat. Res. 2012;470(9):2541–2553. doi: 10.1007/s11999-012-2475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramasubramanian A, Shiigi S, Lee GK, Yang F. Non-viral delivery of inductive and suppressive genes to adipose-derived stem cells for osteogenic differentiation. Pharm. Res. 2011;28(6):1328–1337. doi: 10.1007/s11095-011-0406-9. [DOI] [PubMed] [Google Scholar]
- 34. Andersen MO, Nygaard JV, Burns JS, et al. siRNA nanoparticle functionalization of nanostructured scaffolds enables controlled multilineage differentiation of stem cells. Mol. Ther. 2010;18(11):2018–2027. doi: 10.1038/mt.2010.166. ▪ Presents biodegradable nanostructured polycaprolactone scaffolds coated with siRNA-embedded nanoparticles to direct mesenchymal stem cell differentiation for cell therapy. Different tissue types were developed by placing different siRNA-embedded particles into spatially distinct locations within a single implant.
- 35.Leachman SA, Hickerson RP, Schwartz ME, et al. First-in-human mutation-targeted siRNA Phase Ib trial of an inherited skin disorder. Mol. Ther. 2010;18(2):442–446. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang G, Guo B, Wu H, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012;18(2):307–314. doi: 10.1038/nm.2617. ▪ Liposomal delivery system using a surface-conjugated peptide (AspSerSer)6 to successfully deliver siRNA to bone-forming surfaces. Increased bone formation, and enhanced bone micro-architecture and mass were obtained in both wild-type and ovarectomized rats.
- 38.Wang D, Miller SC, Shlyakhtenko LS, et al. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjug. Chem. 2007;18(5):1375–1378. doi: 10.1021/bc7002132. [DOI] [PubMed] [Google Scholar]
- 39.Yarbrough DK, Hagerman E, Eckert R, et al. Specific binding and mineralization of calcified surfaces by small peptides. Calcif. Tissue Int. 2010;86(1):58–66. doi: 10.1007/s00223-009-9312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manaka T, Suzuki A, Takayama K, Imai Y, Nakamura H, Takaoka K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials. 2011;32(36):9642–9648. doi: 10.1016/j.biomaterials.2011.08.026. ▪ A hydrogel delivery system carrying siRNA targeting the knockdown of a major antagonist to BMPs showed enhanced endogenous BMP bioavailability via local targeting. Induced bone formation resulting from this method broadens the scope of choosing siRNA targets from bone remodeling signaling pathways.
- 41.Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res. Ther. 2008;10(3):R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Tran KK, Shen H, Grainger DW. Selective local delivery of RANK siRNA to bone phagocytes using bone augmentation biomaterials. Biomaterials. 2012;33(33):8540–8547. doi: 10.1016/j.biomaterials.2012.07.039. ▪ Presents a local siRNA targeting strategy to osteoclasts and their precursors as professional phagocytes using micron-sized degradable polylactic-co-glycolic acid particles containing siRNA. These particles are embedded within clinical familiar bone augmentation material used in osteoporosis: calcium phosphate cement.