Figure 3.
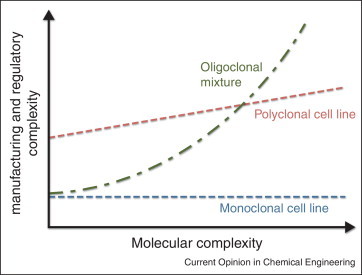
Comparison of costs associated with manufacturing and development of antibody combinations. Costs are fixed for production of a single monoclonal or bispecific antibody, since just one master cell line, manufacturing process and clinical trial is required. In contrast, for oligoclonal cocktails, manufacturing costs increase linearly with the number of unique antibodies processed in parallel, while regulatory costs increase exponentially since all pairwise combinations must be assessed for safety. For a recombinant polyclonal therapeutic, the initial manufacturing and regulatory costs are high to establish the process, but increase incrementally with further increases in molecular complexity [80••].
