Abstract
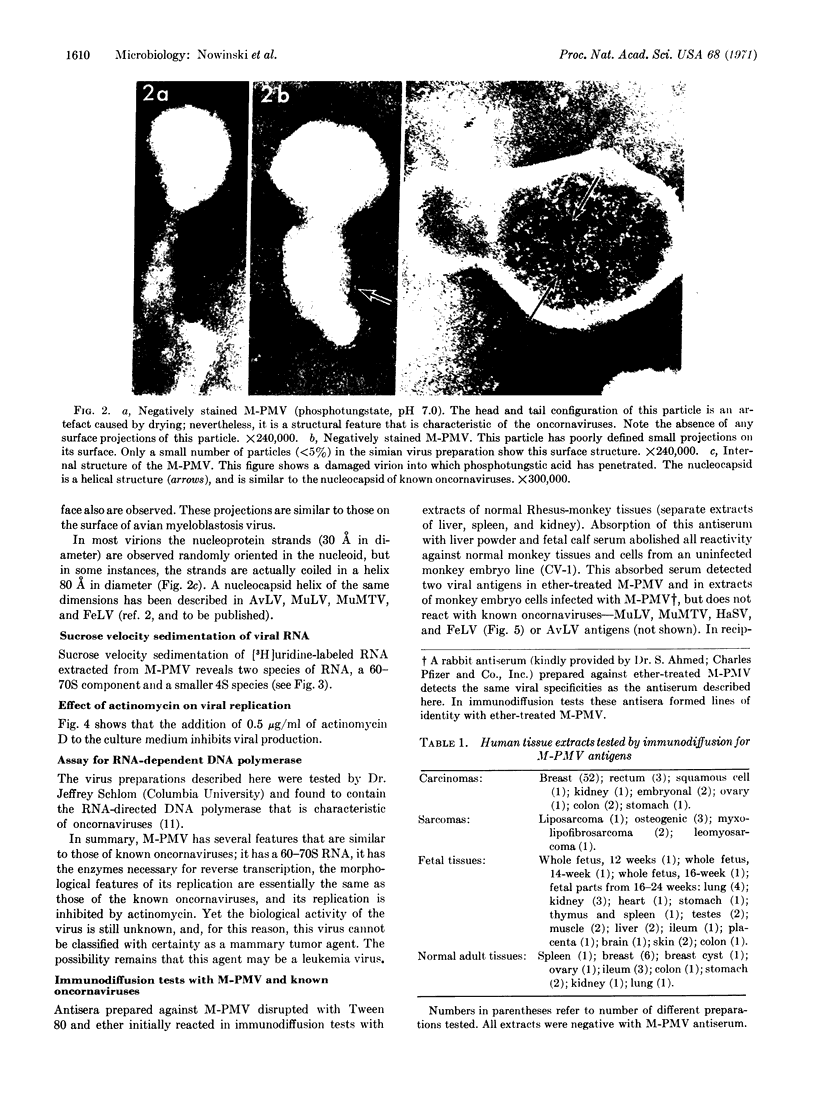
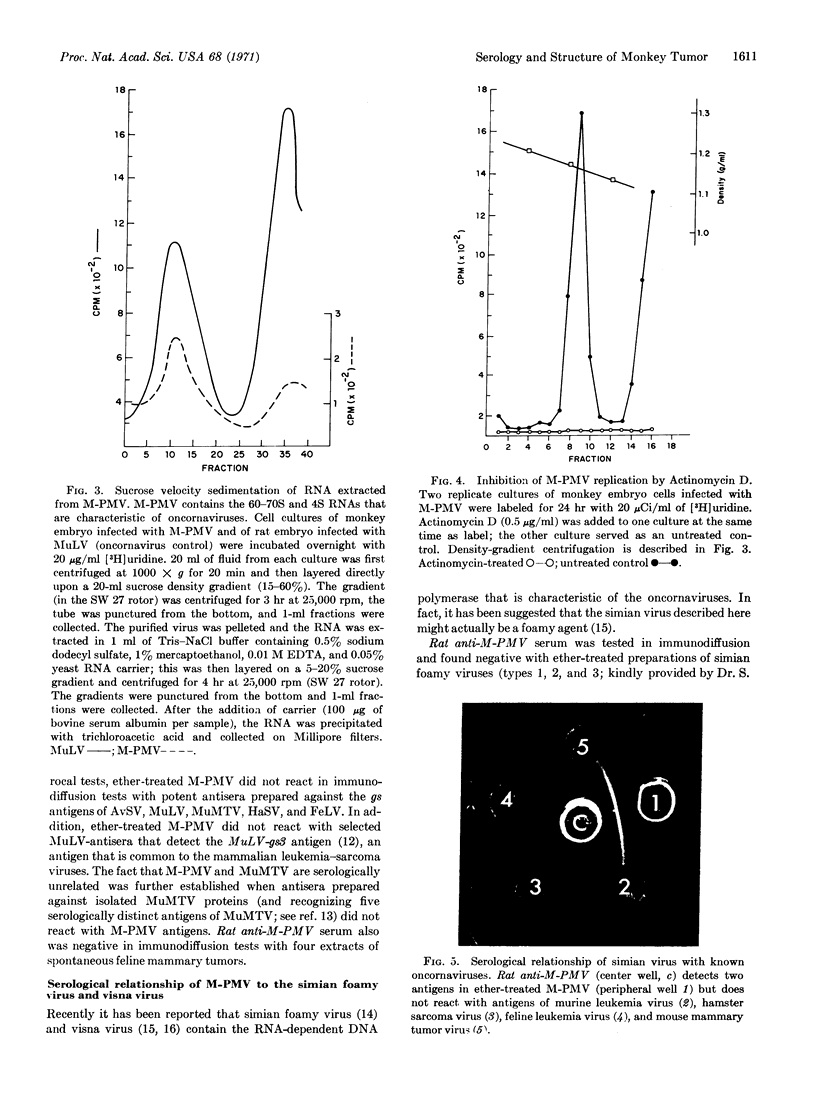
We describe here serological and structural properties of a virus, Mason-Pfizer Monkey Virus (M-PMV), isolated from a simian mammary tumor. This virus is morphologically similar to the known oncogenic RNA viruses (oncornaviruses). It has a 60-70S RNA, and its replication is inhibited by actinomycin. Antisera prepared against the virus isolated by density-gradient centrifugation identify at least two viral structural antigens. Immunodiffusion studies show that this virus is serologically unrelated to three types of simian foamy viruses, visna virus, and the known oncornaviruses. Immunofluorescence reveals that the structural proteins of the virus are synthesized cytoplasmically. Although M-PMV productively infects human cells in vitro, serological analysis does not show the presence of M-PMV antigens in human neoplasia.
Keywords: primate cancer, oncogenic RNA virus, simian foamy virus, visna virus, viral antigens
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Jainchill J. L., Todaro G. J. Murine sarcoma virus transformation of BALB-3T3 cells: lack of dependence on murine leukemia virus. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1236–1243. doi: 10.1073/pnas.66.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Geering G., Aoki T., Old L. J. Shared viral antigen of mammalian leukaemia viruses. Nature. 1970 Apr 18;226(5242):265–266. doi: 10.1038/226265a0. [DOI] [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., ARMSTRONG D., OKUYAN M., SARMA P. S., TURNER H. C. SPECIFIC COMPLEMENT-FIXING VIRAL ANTIGENS IN HAMSTER AND GUINEA PIG TUMORS INDUCED BY THE SCHMIDT-RUPPIN STRAIN OF AVIAN SARCOMA. Proc Natl Acad Sci U S A. 1964 May;51:742–750. doi: 10.1073/pnas.51.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Geering G., Old L. J., DE Harven E., Brody R. S., McDonough S. Feline leukemia virus: occurrence of viral antigen in the tissues of cats with lymphosarcoma and other diseases. Science. 1969 Nov 21;166(3908):1019–1021. doi: 10.1126/science.166.3908.1019. [DOI] [PubMed] [Google Scholar]
- Hirshaut Y., Glade P., Moses H., Manaker R., Chessin L. Association of herpes-like virus infection with infectious mononucleosis. Am J Med. 1969 Oct;47(4):520–527. doi: 10.1016/0002-9343(69)90182-x. [DOI] [PubMed] [Google Scholar]
- Kramarsky B., Sarkar N. H., Moore D. H. Ultrastructural comparison of a virus from a Rhesus-monkey mammary carcinoma with four oncogenic RNA viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1603–1607. doi: 10.1073/pnas.68.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Moore D. H., Geering G., Boyse E. A. A soluble antigen of the mammary tumor virus. Virology. 1967 Jan;31(1):1–14. doi: 10.1016/0042-6822(67)90002-5. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., O'Donnell P. V., Sanders F. K. Serological identification of hamster oncornaviruses. Nat New Biol. 1971 Apr 28;230(17):282–284. doi: 10.1038/newbio230282a0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Sarkar N. H., Moore D. H. Common properties of the oncogenic RNA viruses (oncornaviruses). Virology. 1970 Dec;42(4):1152–1157. doi: 10.1016/0042-6822(70)90367-3. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Todaro G. J., Scolnick E. M., Aaronson S. A. RNA dependent DNA polymerase in primate syncytium-forming (foamy) viruses. Nature. 1971 Jan 22;229(5282):258–260. doi: 10.1038/229258a0. [DOI] [PubMed] [Google Scholar]
- Schlom J., Harter D. H., Burny A., Spiegelman S. DNA polymerase activities in varions of visna virus, a causative agent of a "slow" neurological disease. Proc Natl Acad Sci U S A. 1971 Jan;68(1):182–186. doi: 10.1073/pnas.68.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. DNA polymerase activities and nucleic acid components of virions isolated from a spontaneous mammary carcinoma from a rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1613–1617. doi: 10.1073/pnas.68.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. DNA-directed DNA polymerase activity in oncogenic RNA viruses. Nature. 1970 Sep 5;227(5262):1029–1031. doi: 10.1038/2271029a0. [DOI] [PubMed] [Google Scholar]
- Stone L. B., Scolnick E., Takemoto K. K., Aaronson S. A. Visna virus: a slow virus with an RNA dependent DNA polymerase. Nature. 1971 Jan 22;229(5282):257–258. doi: 10.1038/229257a0. [DOI] [PubMed] [Google Scholar]