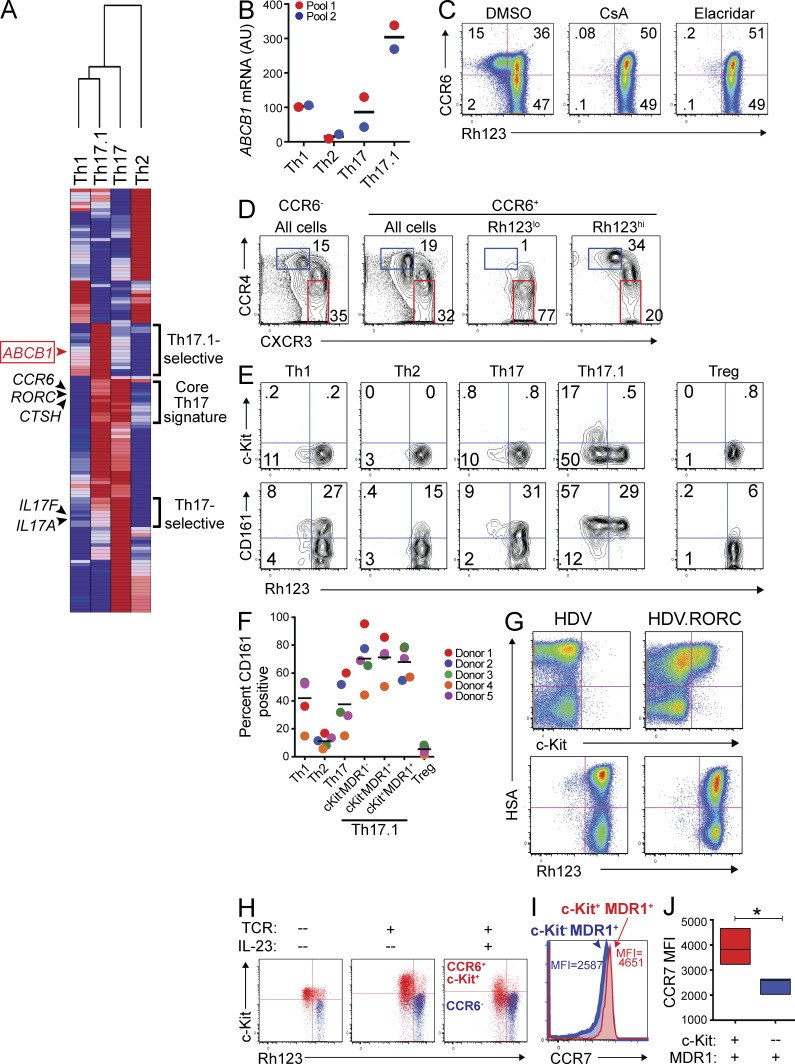
Figure 2.
A novel subset of human Th17.1 cells is characterized by transient c-Kit and stable MDR1 expression. (A) TEM Th1, Th2, Th17, or Th17.1 cells were FACS-sorted from healthy adult donor peripheral blood as in Fig. 1 B. Sorted cells were stimulated with anti-CD3/anti-CD28 for 36 h and RNA was isolated for microarray analysis. Mean normalized raw gene expression values from two independent microarray experiments on cells sorted from different donor pools (each pool containing blood from 3–4 donors) were used to identify differentially expressed genes (1.8-fold cutoff). Data shown is a hierarchical clustering heatmap of all differentially expressed genes, with log2 transformation and row normalization. Red, high relative gene expression; dark blue, low relative gene expression. Representative gene symbols within each cluster are shown (a complete list of the genes and their absolute expression values within each cluster is provided in Table S1). (B) TEM Th1, Th2, Th17, or Th17.1 cells, FACS-sorted as in Fig. 1 B, were stimulated with anti-CD3/anti-CD28 for 72 h and expression of ABCB1 was determined by nanostring. Data are shown as ABCB1 mRNA expression (AU, arbitrary units) in two experiments performed on independent (color-coded) donor pools, with each pool containing blood from 2–4 individual donors. Horizontal bars represent the mean values. (C) Total CD4+CD25−CD45RO+ memory T cells isolated from healthy adult donor peripheral blood were labeled with rhodamine 123 (Rh123). After a 1-h efflux period at 37°C in the presence of vehicle (DMSO) or MDR1 inhibitors (CsA, cyclosporine A; Elacridar, selective MDR1 inhibitor), cells were stained with antibodies against CCR6, and Rh123 efflux and CCR6 expression was analyzed by FACS. Data shown are FACS plots from one experiment performed on cells from a healthy adult donor, and represent 3–4 independent experiments performed on cells isolated from different donors. (D) Rh123 efflux by CD4+ memory T cells was determined by FACS analysis as in C. After Rh123 efflux, cells were stained with antibodies against CCR6, CCR4, and CXCR3, and CCR4 and CXCR3 expression was analyzed on total CCR6− or CCR6+ cells, or on CCR6+ cells gated as Rh123lo (MDR1+) or Rh123hi (MDR1−). Data shown are representative FACS plots of >10 experiments performed on memory T cells isolated from independent donors. (E) CD4+ memory T cells were labeled with Rh123 and analyzed for Rh123 efflux as in C. After Rh123 efflux, cells were stained with antibodies against CD25, CCR6, CCR4, CXCR3, CCR7, c-Kit (CD117), and CD161. Cells were gated as TEM (CCR7lo) Th1 (CD25−CCR6−CCR4loCXCR3hi), Th2 (CD25−CCR6−CCR4hiCXCR3lo), Th17 (CD25−CCR6+CCR4hiCXCR3lo), Th17.1 (CD25−CCR6+CCR4loCXCR3hi), or T reg (CD25hi), and Rh123 efflux versus c-Kit (CD117; top) or CD161 (bottom) expression was analyzed in each subset. The FACS plots shown are representative of 5 independent experiments performed on memory T cells isolated from different donors. (F) The percentage of CD161+ cells within human TEM Th1, Th2, Th17, Th17.1 subsets and T reg cells was determined by FACS analysis as in E. Th17.1 cells were further gated into 3 subsets based on c-Kit expression and Rh123 efflux: c-Kit−MDR1−/Rh123hi, c-Kit−MDR1+/Rh123lo, and c-Kit+MDR1+/Rh123lo. Individual and mean percentages of CD161+ cells within each subset ± SD from 5 independent experiments performed on memory T cells isolated from individual (color-coded) donors is shown. (G) Naive CD4+ T cells were isolated from healthy adult donor peripheral blood, stimulated with anti-CD3/anti-CD28, and transduced with empty- (HDV) or RORC-containing (HDV.RORC) lentiviral particles that also contain a mouse HSA (heat stable antigen; a.k.a. CD24) expression cassette. Transduced T cells were expanded in IL-2–containing media for 7 d, and were then loaded with Rh123, incubated at 37°C for 1 h to allow for Rh123 efflux, and stained with antibodies against c-Kit or mouse HSA; Rh123 efflux and c-Kit expression was analyzed as a function of HSA expression in transduced T cells by FACS. FACS plots shown are representative of 3 independent experiments performed on naive T cells isolated from different donors. (H) CD4+ memory T cells isolated from healthy adult peripheral blood were FACS-sorted into CD25−CCR6+c-Kit+ (red) or CCR6− (blue) subsets. Cells were either left resting (no TCR), or were stimulated with anti-CD3/anti-CD28 and cultured for 6 d with or without IL-23. On day 6, cells were loaded with Rh123, stained with antibodies against c-Kit after Rh123 efflux, and analyzed by FACS. FACS plots shown are representative of 3 experiments using cells sorted from different donor pools, with each pool containing blood from 2–4 individual donors. (I) CD4+ memory T cells isolated from healthy adult peripheral blood were analyzed for Rh123 efflux as in E. CCR7 expression was assessed on c-Kit+ (red histogram) and c-Kit− (blue histogram) MDR1+/Rh123loCD25−CCR6+CCR4loCXCR3hi Th17.1 cells by FACS. The overlaid FACS histogram represents 4 experiments performed on memory T cells isolated from individual donors. CCR7 mean fluorescent intensity (MFI) is shown for c-Kit+ (red text) and c-Kit− (blue text) MDR1+ Th17.1 cells. (J) CCR7 MFI in c-Kit+ (red) and c-Kit− (blue) MDR1+ Th17.1 cells was determined by FACS analysis as in I. Data are shown as mean CCR7 MFI ± SD in c-Kit+ or c-Kit− MDR1+ Th17.1 cells from 4 individual donors. *, P < 0.05 by paired Student’s t test.