Abstract
There are many challenges to performing clinical research in resource-limited settings (RLS). Here we discuss several of the most common laboratory issues that must be addressed. These include issues relating to organization and personnel, laboratory facilities and equipment, standard operating procedures, external quality assurance, shipping, laboratory capacity and data management. While much progress has been made, innovative ways of addressing some of these issues are still very much needed.
Keywords: good laboratory practice, quality management, resource limited settings, hiv infection, tuberculosis
Human immunodeficiency (HIV) and tuberculosis (TB)-related clinical research conducted in resource limited settings (RLS) has the potential to generate data that can quickly lead to improvements in care and treatment in these regions. Laboratory results need to be of the highest quality, primarily to protect the health and safety of the trial participants, but also to provide reliable clinical trial data that will be used as the basis for improving the standard of care. Ensuring appropriate quality management (QM) is vital to performing clinical research in RLS and has proven challenging (1–7). In resource rich countries, like the United States, clinical laboratories operate under regulations imposed by the Clinical Laboratories Improvement Amendments (CLIA) (8) and obtain certification by passing inspections every two years, usually by the College of American Pathologists (CAP), and regular and ongoing participation in external quality assurance (EQA) provided by CAP. Outside the United States, certifications by other organizations like South African National Accreditation System (SANAS) and International Organization for Standardization (ISO) can help to improve laboratory quality assurance. However, irregularities in laboratory consistency (from excellent to minimal QM) have been observed in many projects funded by the Division of AIDS (DAIDS; part of the National Institute of Allergy and Infectious Diseases, National Institutes of Health) and constitute a large obstacle to performing clinical research in RLS.
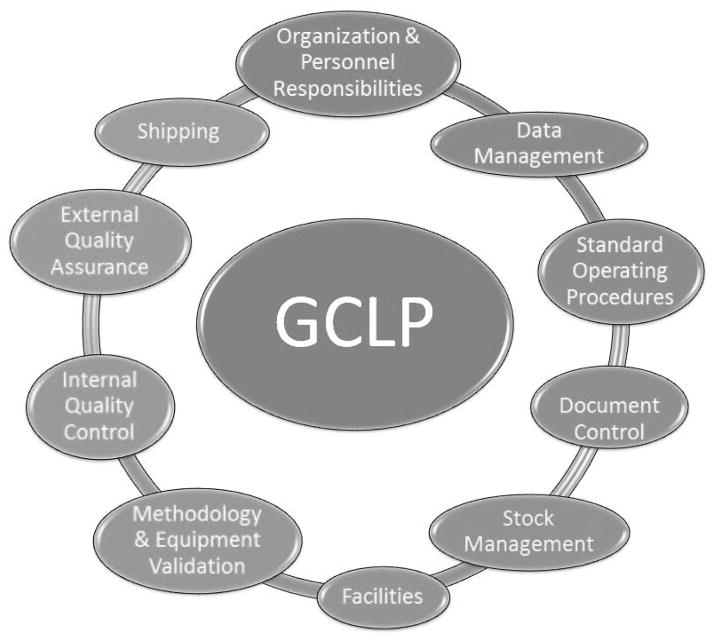
To help address this situation, DAIDS has developed guidelines for clinical laboratories that perform testing for DAIDS-supported clinical research. This document, Good Clinical Laboratory Practice (GCLP) (9, https://www.daidscrss.com/LaboratoryManagementCenter/Pages/GCLP_Standards.aspx), brings together many of the quality management tenets of several groups (CLIA, GCLP, CAP, SANAS). Coupled to the GCLP guidelines, DAIDS has put in place annual laboratory assessments for laboratories performing testing for DAIDS-supported studies. Feedback from these assessments has highlighted common areas requiring additional attention in the laboratory (Figure 1).
Figure 1.
Schematic of the multiple steps involved in running a laboratory that adheres to Good Clinical Laboratory Practice.
To help laboratories performing testing for DAIDS-funded clinical trials comply with GCLP, DAIDS offers support in the form of quality assurance contracts that provide assistance with QM issues. One contract, Patient Safety Monitoring in International Laboratories (pSMILE) was put in place to work with laboratory personnel, mostly through email and conference calls, to help laboratories with standard testing such as chemistry, hematology, serology and other tests (10). The public portion of the website for this contract (www.pSMILE.org) is an open resource with a vast amount of information on laboratory QM available to any laboratory worldwide. Furthermore, support has been put in place for more complex testing, through the Virology Quality Assurance (VQA) contract, which provides laboratory support for HIV-related virological tests (HIV viral load testing, HIV diagnostic PCR testing and HIV drug resistance genotyping). The Immunology Quality Assurance (IQA) contract provides support for CD4 lymphocyte count testing. Other groups have put similar systems in place for remote laboratory oversight with varying levels of success (11, 12). Clearly this type of remote oversight cannot fully substitute for committed oversight at the laboratory, which should include a Management Review Process of all errors, quality control (QC) problems and audit findings.
To ensure high quality laboratory results, organization and personnel responsibilities must be addressed. One of the most difficult problems that laboratories in RLS encounter is the lack of qualified personnel, especially in upper management areas, Laboratory Directors and Laboratory Supervisors. In the US, CLIA regulations define the qualifications of Clinical Laboratory Directors and Supervisors (8). In RLS, people with these qualifications can be very difficult to find and retain, which often results in less-qualified individuals being appointed to these positions who do not have the necessary knowledge and background in QM. Clearly, some laboratories have excellent Directors and Supervisors who are actively involved in the operation of the laboratory, reviewing of QM, overseeing proper training and competency in both QC and tests performed and resolving any problems. These types of laboratories consistently perform well and some have achieved CAP certification. However, in other laboratories, there can be a lack of input from management and technicians are often poorly trained. This is largely a consequence of high staff turnover, as once trained, staff find that they can make more money at a laboratory in another area or another country. This makes it very difficult to keep up with the hiring and continuing education, training and competency evaluation of personnel. Staff retention can be increased by senior management ensuring there is both active supervision and performance management. More importantly, creating an understanding of the importance of the clinical research project, and of the laboratory functions, can help staff feel empowered and important in the development of life-saving therapy, which can help improve staff retention.
Apart from the obstacle of staff retention, laboratory facilities, including laboratory space, equipment, reagents and consumables, are often inappropriate. To help with the problems relating to the supply of reagents and consumables, stock management or inventory systems should be put into place. This should include a mechanism for recording purchasing, checking what is received, and regularly checking the amount of stock required, so that suppliers can be informed well in advance of what is required and can plan accordingly.
Availability of appropriate laboratory equipment and appropriate maintenance can be problematic in RLS. For studies conducted under an Investigational New Drug application (IND), the US Food and Drug Administration (FDA) recommends the use of FDA-approved test methods. However, many laboratories in RLS cannot participate in these studies as the FDA-approved methods are not available, are too expensive, or not well-suited for the region (analyte might be slightly different or based on a different population). Additional verification and validation of unapproved test methods may be necessary, which can cause delays and can result in the laboratory incurring extra expense in order to perform the validation testing. Scheduling any necessary extra validation work well in advance of the clinical trial can help prevent delays.
Some laboratories cannot afford to purchase equipment maintenance agreements. In addition, some companies do not have the staff to adequately cover the requested maintenance calls. This can result in delays with equipment that needs servicing by the company. Scheduling regular maintenance calls with equipment suppliers can help.
To ensure that equipment is operating properly and that laboratories can achieve results consistent with the manufacturer’s specifications, it is important for laboratories to perform validations or verifications on both equipment and assays. However, suppliers often do not provide help with verification or validation of the equipment upon installation, and laboratories often struggle with how to develop an appropriate validation plan and keep the appropriate documentation. The DAIDS contract resources, pSMILE, VQA and IQA all provide support for equipment validation, including providing validation plans and, in the case of VQA and IQA, standards and controls for use in the validations. This has helped the laboratories not only with the performance of validations, but also with instruction on how to perform these types of validations for future reference.
Many RLS still experience problems with electrical power and with internet connections. While many laboratories now have access to a power generator that can provide power to instruments during an outage, not all equipment is connected to this back-up power and there may be delays between the time the power goes out and the time the generator power starts. Any assays being run at this time may be compromised. This can also impact on the quality of the samples being stored for further studies at the site. The laboratory assessment now includes checking for the presence of a generator and documentation of generator function, which has helped somewhat in this area. Internet connectivity problems can prevent laboratories from communicating with above mentioned resources, sending results electronically, and from viewing documents and training materials online. Many laboratories have been unable to access online modules for GCLP refresher training due to these problems. In some cases the documents and materials can be sent on CD-ROM for use in the laboratory, but other problems with connectivity remain.
Laboratories often lack well-written Standard Operating Procedures (SOPs). These are vital to ensure all techniques and processes in the laboratory are standardized, thereby contributing to reproducibility and ensuring good quality data. Regular review, training and competency assessment of staff on the SOPs is also a frequent obstacle in RLS. The GCLP document (https://www.daidscrss.com/LaboratoryManagementCenter/Pages/GCLP_Standards.aspx) includes instruction on development, implementation and continual review of SOPs.
Many laboratories do not perform external quality assurance (EQA) or have inconsistent EQA results for tests performed. For DAIDS-supported clinical trials, the pSMILE contract ensures that laboratories are performing appropriate EQA for standard laboratory testing including chemistry, hematology, serology and other tests. The VQA contract provides an EQA program for HIV virological tests and the IQA contract provides an EQA program for CD4 testing. All of these contracts also track the EQA performance of laboratories and help with investigating and resolving EQA and QC failures.
Shipping delays for test kits and critical reagents are a common problem in RLS (6, 10). Often, test kits and controls are delayed in customs for so long that their shelf life is very short once they get to the laboratory. This is extremely problematic for some laboratories and often results in delayed testing or work stoppage due to the inability to receive kits and reagents within their expiration dates. Some laboratories have found creative ways of getting these items through customs in a timely manner, but many laboratories still experience problems. Many clinical trial protocols require specimens to be shipped to central laboratories outside the country, often to perform specialized testing not performed by the laboratory, or when consistency of having a single laboratory perform the test is desirable in order to minimize laboratory-to-laboratory variation in the results. Shipping of samples can be a major obstacle for some laboratories. Prior to shipping, material transfer agreements and export/import permits need to be obtained, which can result in long delays to shipments. Some countries will not allow shipping of certain samples as the country would like to see capacity developed in-country, and sometimes due to concerns over how the samples will be used. Some countries allow samples to be shipped only to a specialty laboratory within the region and not to a central laboratory in the United States or Europe. This can result in additional costs and delays in testing. The expense of shipping is usually covered by the clinical trial budget; however, laboratories need to have personnel trained in the international shipping of clinical samples, some of which must be shipped under the IATA designation of “dangerous goods.”
Finally, laboratory capacity can sometimes be problematic, as many laboratories in RLS perform both clinical research and provide a service for routine patient management. In these high-volume laboratories, it is easy for the laboratory personnel to pay less attention to QM, due to the need to keep up with the very large volume of testing. Procedures must be in place to ensure QM is followed not only for the clinical research samples, which often represent only a minority of samples being processed in the laboratory, but also for routine samples. Committed Laboratory Directors and Managers are critical in these laboratories. In addition, to organize the QM and further aid the staff workload, appropriate data management systems need to be put in place. These systems should ensure patient confidentiality while also ensuring that information is easily accessible to appropriate laboratory personnel. To make this a useful tool for the laboratory staff, training on the data management system is essential.
There are many hurdles to doing clinical research in RLS. In this paper we point out the most common laboratory-associated problems we have experienced. Progress has been made in alleviating several of these problems; however, improvements can still be achieved. One of the most effective methods of dealing with the majority of these problems is for the clinical investigators to establish a close working relationship with the Laboratory Director and Supervisor to ensure they are committed to the process and that QM systems are in place and monitored on a regular basis. In this way, a culture of laboratory quality is promoted and seen as valuable to everyone involved in clinical research. This will develop an infrastructure that will allow laboratory QM to remain in place for many years, helping these laboratories to provide quality test results for their patients in need.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported in part by National Institutes of Health grant UM1AI068636-07 from the National Institute of Allergy and Infectious Diseases to CW
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Elbireer AM, Jackson JB, Sendagire H, et al. The good, the bad, and the unknown: quality of clinical laboratories in Kampala, Uganda. PLoS One. 2013;8:e64661. doi: 10.1371/journal.pone.0064661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olmsted SS, Moore M, Meili RC, et al. Strengthening laboratory systems in resource limited settings. Am J Clin Pathol. 2010;134:374–380. doi: 10.1309/AJCPDQOSB7QR5GLR. [DOI] [PubMed] [Google Scholar]
- 3.Abimiku AG. Building laboratory infrastructure to support scale-up of HIV/AIDS treatment, care, and prevention: in-country experience. Am J Clin Pathol. 2009;131:875–886. doi: 10.1309/AJCPELMG6GX6RQSM. [DOI] [PubMed] [Google Scholar]
- 4.Guindo MA, Shott JP, Saye R, et al. Promoting good clinical laboratory practices and laboratory accreditation to support clinical trials in Sub-Saharan Africa. Am J Trop Med Hyg. 2012;86:573–579. doi: 10.4269/ajtmh.2012.11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petti CA, Polage CR, Quinn TC, et al. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim F, Dosoo D, Kronmann KC, et al. Good clinical laboratory practices improved proficiency testing performance at clinical trials centers in Ghana and Burkina Faso. PLoS One. 2012;7:e39098. doi: 10.1371/journal.pone.0039098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birx D, de Souza M, Nkengasong JN. Laboratory challenges in the scaling up of HIV, TB, and malaria programs: the interaction of health and laboratory systems, clinical research, and service delivery. Am J Clin Pathol. 2009;131:849–851. doi: 10.1309/AJCPGH89QDSWFONS. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Laboratory Improvement Amendments (CLIA) [Accessed June 27, 2013.];n.d Available at: http://wwwn.cdc.gov/clia/default.aspx.
- 9.Ezzelle J, Rodriguez-Chavez IR, Darden JM, et al. Guidelines on good clinical laboratory practice: Bridging operations between research and clinical research laboratories. J Pharm Biomed Anal. 2008;46:18–29. doi: 10.1016/j.jpba.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amukele TK, Michael K, Hanes M, et al. External quality assurance performance of clinical research laboratories in Sub-Saharan Africa. AJCP. 2012;138:720–723. doi: 10.1309/AJCP8PCM4JVLEEQR. [DOI] [PubMed] [Google Scholar]
- 11.Crucitti T, Fransen K, Maharaj R, et al. Obtaining valid laboratory data in clinical trials conducted in resource diverse settings: lessons learned from a microbicide phase III clinical trial. PLoS One. 2010;5:e13592. doi: 10.1371/journal.pone.0013592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manyazewal T, Paterniti AD, Redfield RR, et al. Role of secondary level laboratories in strengthening quality at primary level health facilities’ laboratories: an innovative approach to ensure accurate HIV, tuberculosis, and malaria test results in resource-limited settings. Diagn Microbiol Infect Dis. 2013;75:55–59. doi: 10.1016/j.diagmicrobio.2012.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.