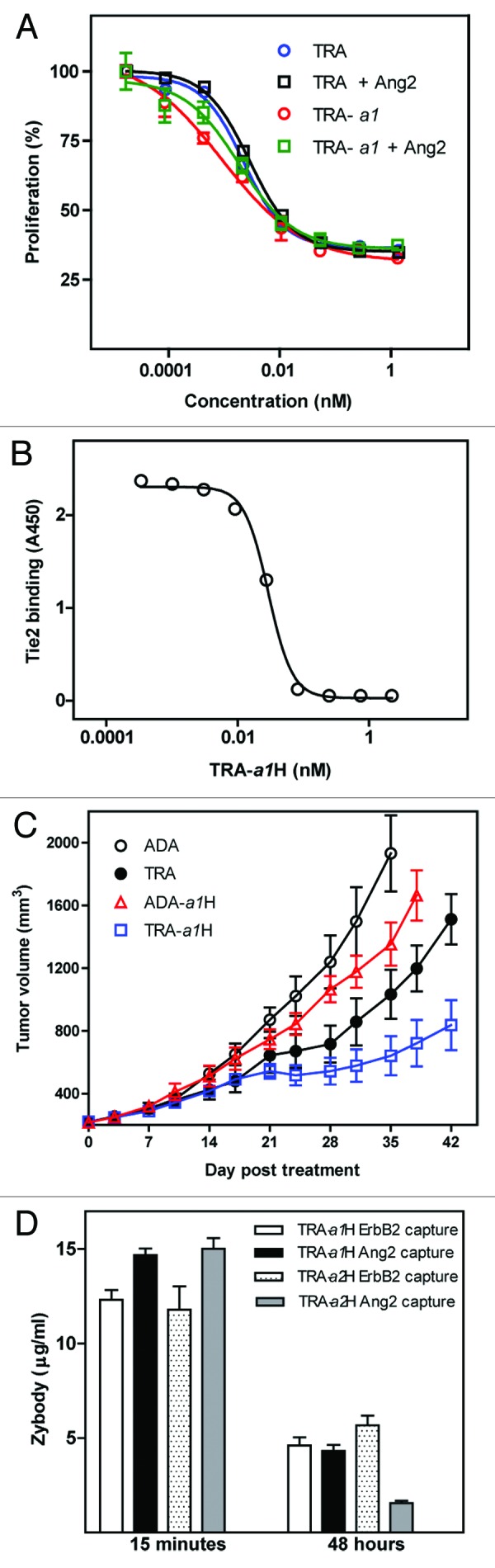
Figure 5. TRA-a1H in vitro and in vivo efficacy. (A) Inhibition of SKBR3 cell proliferation by trastuzumab (TRA) and TRA-a1H was measured in the presence and absence of Ang2. (B) The inhibition of Ang2 binding to Tie2 by TRA-a1H was measured by ELISA. Data represents mean and standard deviation of duplicate samples. (C) The in vivo efficacy of TRA-a1H was assessed in a xenograft tumor model sensitive to the independent inhibition of ErbB2 and Ang2. Mice with pre-established SK-OV-3 tumors were treated with adalimumab (ADA) as a negative control, trastuzumab (TRA), the bispecific zybodies targeting ErbB2 and Ang2 (TRA-a1H) or TNFα and Ang2 (ADA-a1H). P value for Mann Whitney t-test comparing TRA-a1H to trastuzumab at day 42 is 0.007. (D) The in vivo stability of individual target binding moieties on a zybody was measured in CD1 mice after a single injection of trastuzumab-based, bispecific zybodies containing either the a1 or the a2 MRD. Serum samples were collected at 15 min and 48 h and were assayed for mAb scaffold binding (ErbB2 capture) or MRD binding (Ang2 capture) in ELISA assays.
