Abstract
We report on a rare case of sarcoidosis that developed after chemotherapy for ovarian cancer, and mimicked a cancer metastasis. A 52-year-old female diagnosed with stage III ovarian cancer underwent curative surgery and postoperative chemotherapy. Four months later, her whole-body positron emission tomography and computed tomography (CT) scan showed high uptake in the mediastinal lymph nodes, and ovarian cancer recurrence was suspected. Biopsy of the mediastinal lymph nodes and subcutaneous nodules revealed noncaseating granulomas. These lesions resolved spontaneously without treatment; however, newly developed perilymphatic and centrilobular nodules were observed on follow-up chest CT. Surgical biopsy of these lesions also showed noncaseating granulomas. She was finally diagnosed with sarcoidosis.
Keywords: Sarcoidosis, Ovarian neoplasms, Drug therapy, Positron-emission tomography and computed tomography
Introduction
Sarcoidosis is a systemic disorder of unknown etiologycharacterized by widespread development of noncaseating epithelioid cell granulomas in more than one organ system [1]. A review of the literature suggests that the relationship between sarcoidosis and malignancy is controversial; however, new onset sarcoidosis has been observed repeatedly and reported in oncology patients [2-4]. However, few cases of sarcoidosis after chemotherapy have been reported in the literature. We report on a case of sarcoidosis mimicking cancer recurrence, which developed after chemo-therapy for ovarian cancer.
Case Report
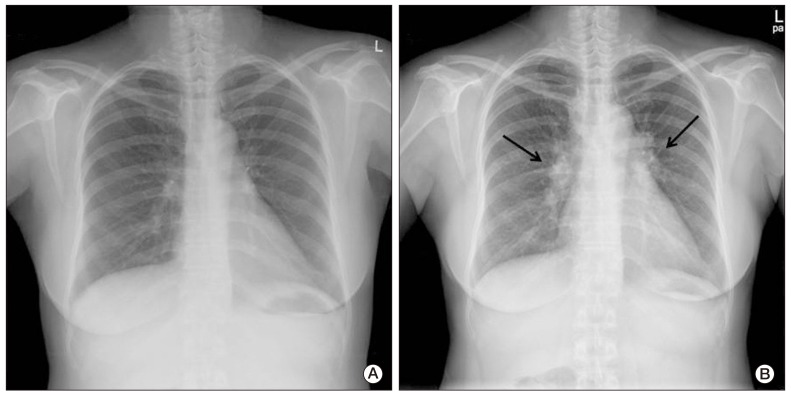
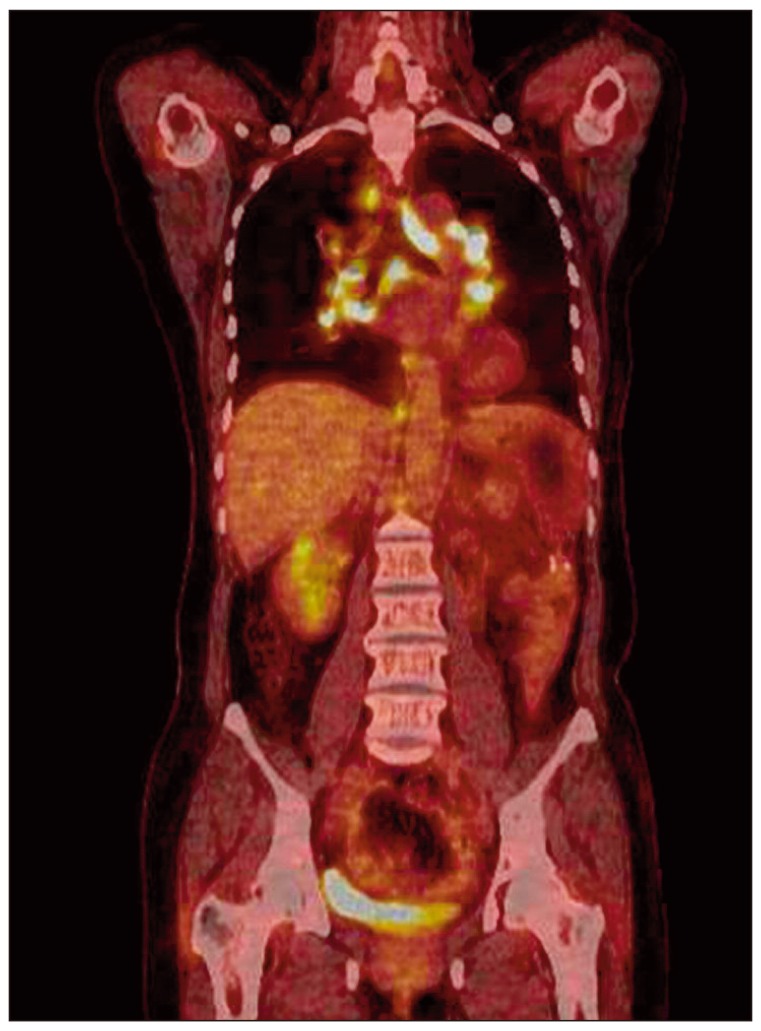
A 52-year-old female was admitted to our hospital with a one-month history of abdominal pain on the left side. Her abdominal computed tomography (CT) scan showed ascites, omental caking, and peritoneal thickening. She underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy. The final diagnosis was stage III ovarian cancer (serous papillary cystadenocarcinoma). She received combination chemotherapy comprising paclitaxel 175 mg/m2+carboplatin 400 mg/m2 for eight cycles for seven months after surgery. There were few serious complications during chemotherapy. However, four months later, she presented with a dry cough, and multiple painless subcutaneous nodules that had developed on her right arm and leg. A plain chest radiograph showed bilateral hilar enlargement compared with a previous film (Fig. 1A and B). Whole-body positron emission tomography and computed tomography (PET/CT) scan for evaluation of cancer recurrence showed no uptake in the subcutaneous nodules, however, multiple areas of high uptake were observed in the enlarged paratracheal, left supraclavicular, retrocrural, and diaphragmatic lymph nodes (the maximum standardized uptake value [SUVmax] of the lymph nodes ranged from 12.3 to 21.5) (Fig. 2) and cancer recurrence in these areas was diagnosed clinically. The same features were observed on both chest CT scan and PET/CT (Fig. 3A). However, her serum levels of carbohydrate antigen 125 (CA125) and carbohydrate antigen 19-9 (CA19-9) were unremarkable; CA125 level was 9.42 U/mL (reference range, 0 to 35.0 U/mL) and CA19-9 level was 3.12 U/mL (referencerange, 0 to 37 U/mL).
Fig. 1.
Plain chest radiograph. (A) Plain chest radiograph before chemotherapy showed no active lung lesion. (B) Plain chest radiograph after chemotherapy showed bilateral hilar enlargement (arrows).
Fig. 2.
Positron emission tomography and computed tomography (PET/CT) images of the lymph nodes. PET/CT scan showed multiple areas of high uptake in the enlarged paratracheal, left supraclavicular, retrocrural, and diaphragmatic lymph nodes.
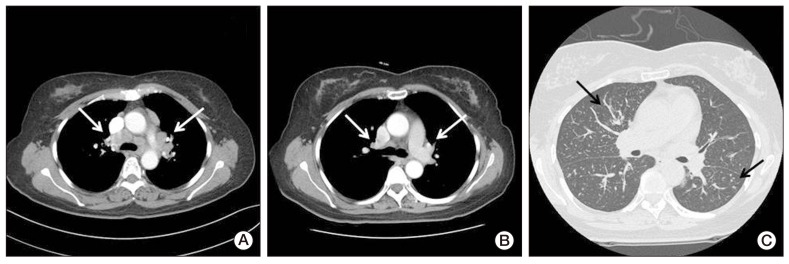
Fig. 3.
Computed tomography (CT) images of the lymph nodes (A) four months after chemotherapy and (B, C) at the follow-up. (A) CT scan of the mediastinal setting showed multiple mediastinal lymph adenopathy (arrows). (B) CT scan of the mediastinal setting showed decreased size of multiple mediastinal lymph nodes (arrows). (C) CT scan of the lung setting showed newly developed perilymphatic and centrilobular nodules in both lungs (arrows).
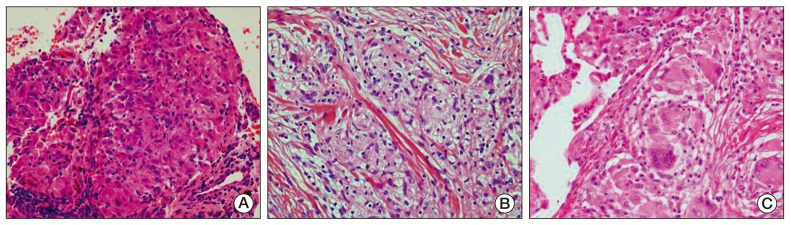
To be certain of the diagnosis of cancer recurrence, biopsy of the mediastinal lymph nodes using endobronchial ultrasound-guided transbronchial needle aspiration and biopsy of the subcutaneous nodules were performed under general anesthesia. The pathological findings showed noncaseating granulomas with no evidence of malignancy (Fig. 4A and B). Ziehl-Neelsen and polymerase chain reaction detection of Mycobacterium tuberculosis in tissues were negative for mycobacterial infection, and a serum interferon-gamma release assay test was also negative. The serum level of angiotensin-converting enzyme (ACE) was 101.4 U/L (reference range, 9 to 47 U/L).
Fig. 4.
Biopsy specimens showed multiple noncaseating granulomas with no evidence of malignancy. (A) Right paratracheal lymph node. (B) Subcutaneous nodule in the right arm. (C) Lung parenchyma (A-C, H&E staining, ×400).
She was diagnosed with sarcoidosis in the lymph nodes and skin. She had mild symptoms, including intermittent dry cough, and no specific treatment was required. She was observed closely at our clinic for three months, and the multiple painless subcutaneous nodules resolved spontaneously. Her follow-up chest CT scan showed an interval decrease in the size of multiple mediastinal lymph nodes (Fig. 3B). However, newly developed perilymphatic and centrilobular nodules were detected in both lungs on the same CT scan (Fig. 3C). Lymphangitic metastasis of ovarian cancer was suspected radiologically, therefore, she underwent video-assisted thoracoscopic wedge resection of the nodules in the right upper lobe. However, the pathological findings of the lung nodules showed noncaseating granuloma, which was similar to that of the mediastinal lymph nodes and skin (Fig. 4C). She was finally diagnosed with sarcoidosis. She was observed closely at our clinic.
Discussion
To the best of our knowledge, this is the first report of sarcoidosis that occurred following surgery and chemotherapy for ovarian cancer in South Korea. In addition, this case demonstrates that the clinical presentation and radiological examinations, including a PET/CT scan, may not be able to discriminate between cancer recurrence and sarcoidosis.
According to the literature, 4-14% of all patients with malignancy can exhibit histopathological evidence of sarcoidosis [5,6]. In oncology patients, noncaseating granulomas have been observed most frequently in the lymph nodes draining the cancer [7]. In the current case, development of noncaseating granulomas occurred not only in the mediastinal lymph nodes, but also in the subcutaneous tissue and lung parenchyma in the absence of recurrence of ovarian cancer, which led to diagnosis of sarcoidosis. It is difficult to explain why the reaction occurred only in distant lymph nodes and organs and not in regional lymph nodes.
Understanding of the pathogenesis of sarcoidosis associated with cancer remains incomplete. According to one hypothesis, localized sarcoidosis may be related to degenerative and necrotic changes within the tumor itself and that systemic sarcoidosis at sites not involved with the tumor may be mediated by humoral and T cell-mediated factors, and subsequent recruitment and activation of macrophages [5,8].
Development of sarcoidosis during or immediately after chemotherapy is rare and has been reported only in a limited number of cases of malignant lymphoma, osteosarcoma, and solid tumors [7,9-11]. The pathophysiological mechanisms of sarcoidosis during or after high-dose chemotherapy are unknown because chemotherapy inhibits the local immunological responses there by preventing granuloma formation and development of associated disease symptoms [9]. It has been postulated that sarcoidosis can be caused by a microorganism because immunosuppression induced by antineoplastic therapy might spread this agent, leading to an immune response resulting ingranuloma formation [9].
This phenomenon has been associated with several different chemotherapeutic agents; the most common causative agent is the biologic modifier alpha-interferon [12]. There is only one previous report on development of sarcoidosis associated with combination chemotherapy paclitaxel and carboplatin [13]. Umezu et al. [13] reported a case of sarcoidosis that developed during induction chemotherapy comprising paclitaxel and carboplatin for lung cancer. In the current case, development of sarcoidosis occurred four months after chemotherapy; however, the patient described by Umezu et al. [13] was diagnosed with sarcoidosis during chemotherapy. Paclitaxel and carboplatin may have induced the reaction, however, conduct of further studies will be required in order to explain the pathogenesis of sarcoidosis during or after chemotherapy.
As in the current case, discrimination between sarcoidosis and metastasis with a CT scan can be difficult. In addition, the current case showed higher uptakes in the PET/CT scan (e.g., SUVmax of lymph nodes, 12.3 to 21.5) than in previous reports [14,15], suggesting that the SUVmax in the PET/CT scan is not accurate for distinguishing between sarcoidosis and malignancy. Therefore, evaluation of other clinical parameters or the serum ACE level might be necessary in order to discriminate between sarcoidosis and metastasis.
In conclusion, when cancer recurrence is suspected despite successful chemotherapy for a neoplastic disease, the clinician should consider the possibility of sarcoidosis even if it occurs during or immediately after treatment. Histopathological examinations can be useful for making a precise diagnosis and for appropriate decision making.
Acknowledgments
This work was supported by a biomedical research grant from Pusan National University Hospital (2012).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 2.Caras WE, Dillard T, Baker T, Pluss J. Coexistence of sarcoidosis and malignancy. South Med J. 2003;96:918–922. doi: 10.1097/01.SMJ.0000083858.65141.79. [DOI] [PubMed] [Google Scholar]
- 3.Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605–613. doi: 10.1378/chest.107.3.605. [DOI] [PubMed] [Google Scholar]
- 4.Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247–251. doi: 10.1038/bjc.1974.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13:147–156. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 6.Llombart A, Jr, Escudero JM. The incidence and significance of epithelioid and sarcoid-like cellular reaction in the stromata of malignant tumours:a morphological and experimental study. Eur J Cancer. 1970;6:545–551. doi: 10.1016/0014-2964(70)90076-9. [DOI] [PubMed] [Google Scholar]
- 7.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326–333. doi: 10.1016/j.clindermatol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Tangen JM, Naess A, Aasen T, Morild I. Non-caseating granulomas in patients with hematologic malignancies: a report of three cases. Acta Med Scand. 1988;223:83–87. doi: 10.1111/j.0954-6820.1988.tb15769.x. [DOI] [PubMed] [Google Scholar]
- 9.Kornacker M, Kraemer A, Leo E, Ho AD. Occurrence of sarcoidosis subsequent to chemotherapy for non-Hodgkin's lymphoma: report of two cases. Ann Hematol. 2002;81:103–105. doi: 10.1007/s00277-001-0415-6. [DOI] [PubMed] [Google Scholar]
- 10.Sybert A, Butler TP. Sarcoidosis following adjuvant high-dose methotrexate therapy for osteosarcoma. Arch Intern Med. 1978;138:488–489. [PubMed] [Google Scholar]
- 11.Whittington R, Lazarus A, Nerenstone S, Martin A. Sarcoidosis developing during therapy for breast cancer. Chest. 1986;89:762–763. doi: 10.1378/chest.89.5.762. [DOI] [PubMed] [Google Scholar]
- 12.Sacchi S, Kantarjian H, O'Brien S, Cohen PR, Pierce S, Talpaz M. Immune-mediated and unusual complications during interferon alfa therapy in chronic myelogenous leukemia. J Clin Oncol. 1995;13:2401–2407. doi: 10.1200/JCO.1995.13.9.2401. [DOI] [PubMed] [Google Scholar]
- 13.Umezu H, Chida M, Inoue T, Araki O, Tamura M, Tatewaki M, et al. Sarcoidosis development during induction chemotherapy for lung cancer mimicked progressive disease. Gen Thorac Cardiovasc Surg. 2010;58:434–437. doi: 10.1007/s11748-009-0549-3. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury FU, Sheerin F, Bradley KM, Gleeson FV. Sarcoid-like reaction to malignancy on whole-body integrated (18)F-FDG PET/CT: prevalence and disease pattern. Clin Radiol. 2009;64:675–681. doi: 10.1016/j.crad.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949–1953. doi: 10.1378/chest.07-1178. [DOI] [PubMed] [Google Scholar]