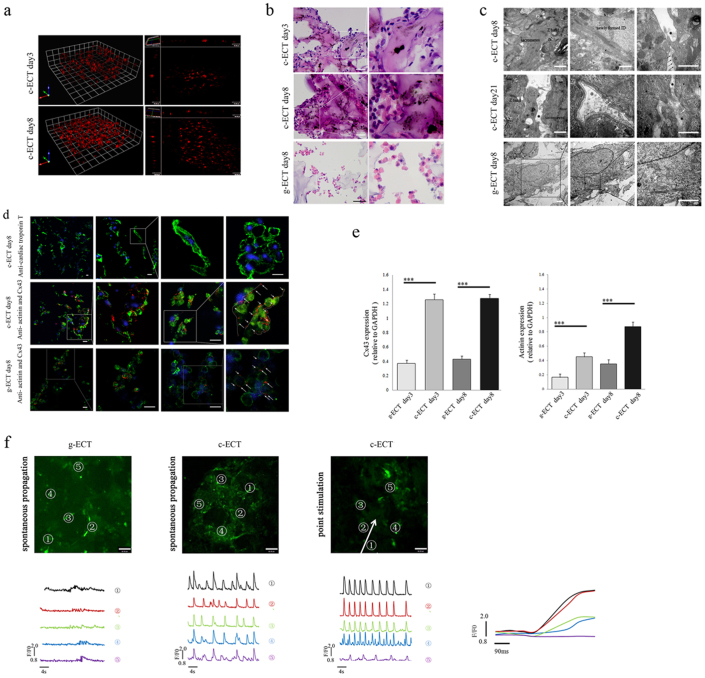
Figure 2. Construction and evaluation of c-ECTs.
(a), 3D live cell imaging showed the process of cell proliferation and migration into SWNT/gelatin hydrogels to organize cardiac tissues from day3 to day8. (b), H&E staining of the c-ECTs on day 3 and day 8 revealed a better organized and more compact multi-cellular aggregates within the SWNT/gelatin scaffolds after electrical field stimulation, however, g-ECTs on day 8 remained the constant small sporadic aggregates. (c), TEM showed the ultra-microstructure of c-ECTs at day 8 with apparent oriented sarcomeres, Z bands, newly formed intercalated disc, and directly contact of carbon nanotubes at localized sites of cardiac membrane surface (black arrow), while the cardiomyocytes showed progressively less organized sarcomeres within the gelatin scaffold. After culture for 21 days, the distribution of carbon nanotubes on cardiac membrane became continuous (within black dotted line) with adjacent membranes concavity (white arrow) and vesicles formation (white dotted line). (d), Immunostaining of c-ECTs on day 8 revealed pervasive cTnT expression (green) and Cx43 gap junction protein (red, white arrow and dotted line) were found between sarcomeric actinin positive cardiomyocytes (green) compared with low expressions in g-ECTs. Nuclei were stained blue. (e), Quantification of sarcomeric actinin protein and Cx43 protein expression by western blot. (f), Calcium transient was assessed at specified 5 points by monitoring calcium dye fluorescence (green). c-ECTs displayed apparent spontaneous electrical activity at each sites compared with little spontaneous electrical activity in g-ECTs. After point stimulation, four tested sites (No.1 to No.4) in c-ECTs showed complete synchronism activity from stimulated sites within 300 μm. The white arrow represents the direction of propagation. F/F0 refers to measured fluorescence normalized to background fluorescence. Scar bars, 100 μm (b), 20 μm (d) or 60 μm (f).