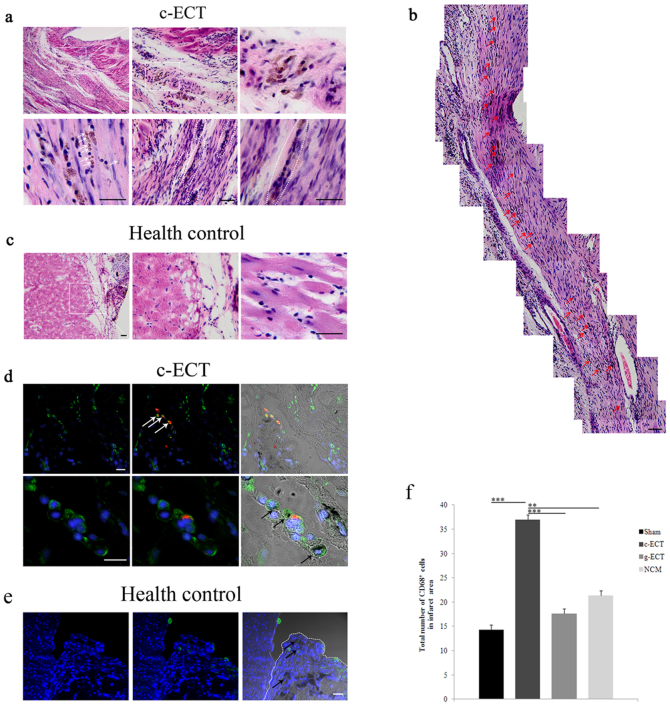
Figure 4. Integration of c-ECTs to host myocardium through Carbon nanotubes migration.
(a), H&E staining of the mid-ventricular 4 week after engraftment showed that carbon nanotubes migrated into the scar areas and located at cell membrane surface or in cytoplasm, as well as intercellular space(white arrow). Carbon nanotubes could connect cells to form dense cell bundle or clusters in the infarct area (white dotted line), even throughout the infarct area (b). Red arrows in (b) showed carbon nanotubes with continuous distribution. (c), Carbon nanotubes were mainly confined to the implants on the surface healthy heart as control. (d), Immunostaining showed CD68 positive macrophages (green) migrated from the c-ECTs to the infarct area, with apparent accumulation in the boundary zone and in scar areas. Some of these macrophages contained phagotrophic carbon nanotubes within their cytoplasm (black arrow), and some of them were c-ECTs-derived DiI+ macrophages (red+ green, white arrow). (e), CD68 positive macrophages (black arrow) were confined to the c-ECTs (white dotted line) on the surface healthy heart as control. Some of these macrophages contained phagotrophic carbon nanotubes within their cytoplasm (black arrow). (f), The number of CD68 positive cells in the infarct areas of c-ECTs compared with g-ECTs, NCM and sham groups. Nuclei are stained blue. Scar bars, 100 μm (a–c) or 20 μm (d, e). Data are mean ± s.e.m. * = p<0.05, ** = p<0.01, *** = p<0.001.