Figure 1.
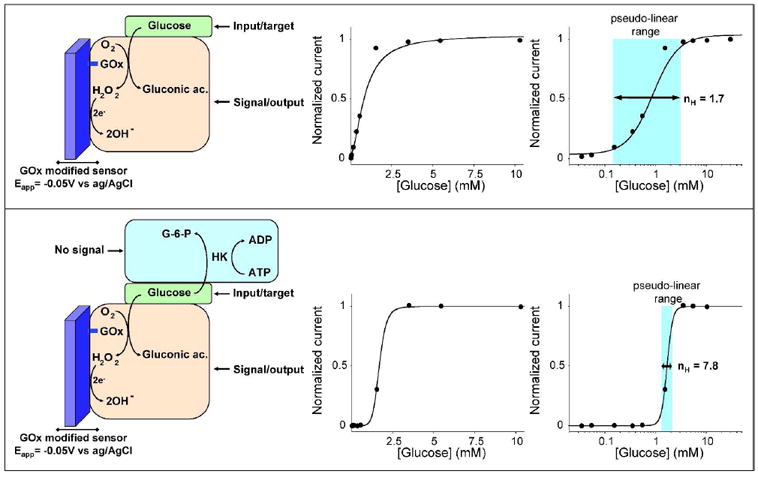
Here we convert the hyperbolic dose-response curve of an enzyme-based sensor into a steep all-or-none digital-like response by employing the “branch point effect”, a mechanism that occurs in some metabolic networks, where two enzymes compete for the same substrate24-26. (top) As our testbed system we have used the well known glucose amperometric sensor, which contains a surface-confined Glucose Oxidase (GOx) that shows a classic Michaelic-Menten response with a Km of 0.8 mM. (bottom) By coupling this enzyme system with another higher affinity competing “depletant” enzyme (here Hexokinase, HK) we can convert the hyperbolic Michaelis-Menten response of GOx into a digital-like output. When the total glucose concentration is equal or lower to the concentration of ATP, all the glucose is converted by HK to glucose-6-phosphate (G-6-P). The glucose is thus sequestered from the GOx, precluding signaling. When the total glucose concentration surpasses the concentration of ATP (the HK catalyzed reaction is saturated), a threshold response is achieved in which further addition of glucose drastically raises its effective concentration. This threshold effect generates a “pseudo-cooperative” dose-response curve in which the output signal arises much more rapidly than would occur in the absence of HK and ATP. In these operative conditions ([ATP]=1.25 mM) the range of glucose concentration at which this sharp transition occurs is compressed to less than 2-fold. The use of the logarithmic scale in the x-axes (right) as opposed to a linear scale (center) renders it easier to evaluate the narrowing of the dose-response curve.
