Figure 4.
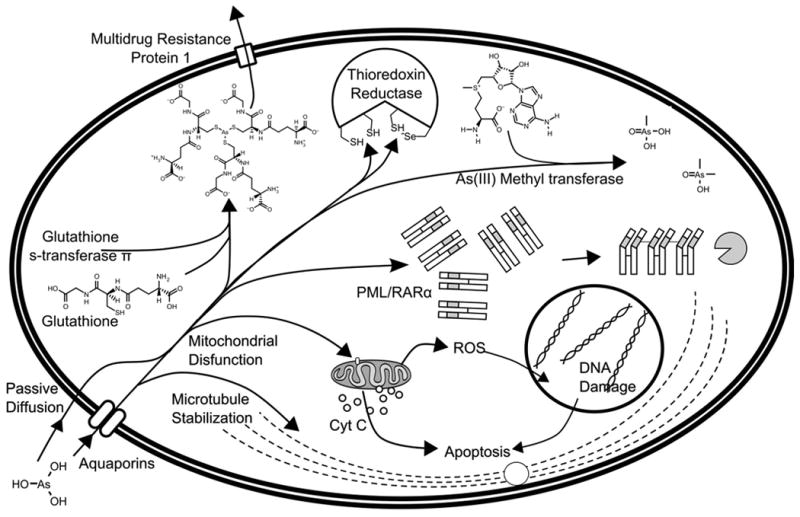
Proposed mechanisms of action for arsenic trioxide. Arsenous acid enters mammalian cells either by passive diffusion, or by the neutral solute transporter proteins in the aquaporin family.51, 52, 55, 56 It then binds to the numerous thiol moieties present in the cell, particularly glutathione, the interaction of which is catalyzed by glutathione s-transferase.65 Arsenous acid can bind to microtubules, preventing depolymerization and halting the cell cycle.79 Arsenic also binds to the active site of thioredoxin reductase, which in turn causes the buildup of oxidized, mis-folded proteins in the cell.78 In acute promyelocytic leukemia, arsenic binds to a zinc finger fusion protein, PML/RARα, which causes aggregation and degradation of the protein.86, 87 Arsenic can be metabolized into methylarsonic acid or dimethylarsinic acid.62 Arsenic interacts with mitochondrial proteins to collapse the mitochondrial membrane potential, produce reactive oxygen species (ROS), release cytochrome c into the cytoplasm and initiate apoptosis.73
