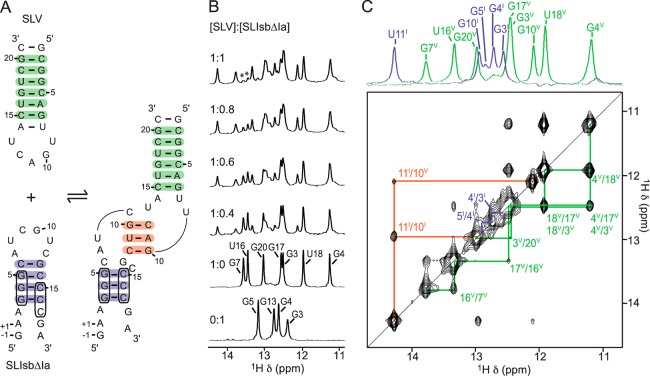
Figure 2.
NMR evidence for formation of the SLIsbΔIa/SLV complex. (A) Proposed secondary structure of SLIsbΔIa, a shiftable SLI variant, and SLV in their free (left) and bound (right) forms. The boxed nucleotides in SLIsbΔIa are those involved in the helix shift associated with SLV binding. (B) Imino region of 1D 1H NMR spectra recorded for the titration of 15N-labeled SLV with unlabeled SLIsbΔIa. 1D NMR spectra were collected under the same conditions and plotted on the same vertical scale but were adjusted to take into account sample dilution during the titration. Imino proton assignment is provided for both SLIsbΔIa and SLV in their free forms. The imino proton signals marked with an asterisk in the 1:1 SLIsbΔIa/SLV complex represent a small excess of free SLV. (C) Imino region of the 2D NOESY spectrum of the SLIsbΔIa/15N-SLV complex. On top, 1D 15N-filtered (purple; SLIsbΔIa) and 15N-edited (green; SLV) spectra of the complex annotated with imino proton assignments. Annotations in (B) and (C) are used to identify imino signals within SLIsbΔIa (purple), within SLV (green), and at the kissing-loop junction (orange), and these imino signals provide evidence for the base pairs shaded with the corresponding colors in (A).