Abstract
The majority of studies examining antigen-presenting cell (APC) function have focused on the capture and presentation of antigens released from pathogens or damaged cells. However, antigen-specific B cells are also capable of efficiently extracting antigens that are either tethered to, or integrally part of the plasma membrane of various target cells. In this study we show that B cells are also highly efficient at extracting integral components of the extracellular matrix (ECM) for subsequent presentation. In particular we demonstrate that B cells specific for aggrecan, an integral component of cartilage ECM, acquire this rheumatoid arthritis candidate autoantigen in both a B-cell-receptor-dependent and a contact-dependent manner. We also demonstrate that the subsequent presentation of aggregan from ECM leads to CD4+ T-cell activation and effector cell formation. Recent studies have identified B-cell-mediated antigen presentation as essential for the development of autoimmunity, but a unique role for B cells compared with other APC has yet to be defined. Our findings lead us to propose that the acquisition of ECM-derived autoantigens represents a mechanism that defines the APC requirement for B cells in the development of autoimmunity.
Keywords: aggrecan, B lymphocyte, CD4+ T lymphocyte, extracellular matrix, rheumatoid arthritis
Introduction
Following the initial description of antigen-presenting cell (APC) function by macrophages,1 and dendritic cells (reviewed in ref. 2), it is now recognized that B-cell APC capacity is also highly efficient3,4 and essential for the generation of T-cell-dependent antibody production.5 More recent studies have shown that B-cell-mediated antigen presentation also represents an essential component of optimal CD4+ T-cell activation per se,6–10 although the reason(s) underlying this remain unknown.
One key feature of B-cell antigen presentation that may set them apart from other APC is the types of antigens that they are capable of acquiring. Unlike other APC, individual B cells express a unique B-cell receptor (BCR), specific for the capture and uptake of a single antigen.3,4,11,12 In addition to the uptake and presentation of both soluble and particulate antigens, the BCR also mediates the extraction and presentation of antigens that are either tethered to, or integrally part of the surface of other target cells.13,14
Understanding the basis of this form of antigen ‘acquisition’ by B cells has important implications for the activation of autoreactive CD4+ T cells and the initiation of autoimmunity. This is compounded by many studies showing that B-cell APC function is an essential component of an increasing number of autoimmune disorders.7,8,15–19 Rheumatoid arthritis (RA) is one such autoimmune disorder, characterized by the presence of self-reactive lymphocytes that mediate the erosion of cartilage and bone,20 where strong evidence exists for the role of B-cell APC function (reviewed in ref. 21). Candidate autoantigens in RA include the two major components of cartilage extracellular matrix (ECM), type II collagen and proteoglycan aggrecan. Knowledge of the biochemistry of assembly and homeostasis of this dynamic environment is considerable, in particular the identification of degradative enzymes involved in cartilage breakdown.22
Aggrecan, which provides loading support to the joint, is attached to the cartilage matrix scaffolding glycosaminoglycan, hyaluronan (HA), via interactions within its N-terminal globular (G)1 domain.23 Interestingly, the enzymatic catabolism responsible for aggrecan turnover results in the sequential release of C-terminal fragments into the synovial fluid with the G1 domain remaining embedded in the cartilage ECM.24 As this domain contains several CD4+ T-cell epitopes including those residues (amino acids 84–103) containing the major arthritogenic epitope,25 its retention within the ECM has presented a paradox with regard to the processes involved in the activation of G1-specific CD4+ T cells. In addition, the removal of the C-terminal glycosaminoglycan side chains increases the immunogenicity of aggrecan in the proteoglycan-induced arthritis (PGIA) model of RA.26
In order to study B-cell APC function in RA, we have recently generated B cells expressing BCR specific for aggrecan (A20-agg). These cells present soluble aggrecan extremely efficiently, requiring approximately 10 000-fold lower levels of antigen than that required by non-specific B cells.27 In the present study we demonstrate that these aggrecan-specific B cells also acquire, in a contact-dependent manner, ECM-immobilized aggrecan leading to the activation of CD4+ T cells specific for the immunodominant G1, p84-103 CD4+ T-cell epitope. These findings offer an explanation for how the major arthritogenic epitope from within the G1 domain of this cartilage autoantigen can be presented to the immune system.
Materials and methods
Cells and culture
The mouse B-cell lymphoma, A20 (IgG2a/κ, H-2d),28 a cloned A20 transfectant (4C5; A20-agg), expressing a human IgG1/κ BCR containing the VH and VL domains from a mouse anti-bovine aggrecan antibody C7.1,27 and the aggrecan-specific, CD4+ T-cell hybridoma 192 (J. Falconer, K. Lowes, A. L. Furmanski, J. Dyson, W. F. Ng, J. R. Robinson, in preparation), H-2Ad restricted, recognizing peptide 84–103 (VVLLVATEGRVRVNSAYQDK) were grown at 37°C in an atmosphere of 5% CO2. All cells were cultured in RPMI-1640 containing 10% fetal calf serum (First Link, Birmingham, UK), 100 μg/ml kanamycin, 2 mm glutamine, 1 mm sodium pyruvate, 100 mm non-essential amino acids, 25 mm HEPES (all Invitrogen Paisley, UK unless stated) and 50 μm β-mercaptoethanol (Sigma-Aldrich, Gillingham, UK). Medium for A20-agg B cells was supplemented with 0·75 mg/ml hygromycin B (Roche, Basel, Switzerland) and 0·5 mg/ml G418. Aggrecan-specific CD4+ T cells (specific for peptide 84–103) were purified from splenocytes isolated from T-cell receptor (TCR)-5/4E8 transgenic mice29 using 10 μl of anti-CD4 microbeads (L3T4; Miltenyi-Biotec, GmbH, Bergisch Gladbach, Germany)/107 splenocytes and magnetized LS columns according to the manufacturer's instructions. Experiments were performed under the terms of the Animals (Scientific Procedures) Act 1986 and were authorized by the Secretary of State, Home Office, UK.
Generation of immobilized aggrecan
Aggrecan isolated from bovine nasal cartilage was purified and deglycosylated as described previously.27 To establish an immobilized form of aggrecan, deglycosylated aggrecan was biotinylated with EZ-link, Sulfo-NHS-LC Biotin (Thermo Fisher Scientific Inc., Waltham, MA) according to the manufacturer's instructions at a molar ratio of 40 : 1. Graded doses of biotinylated aggrecan were incubated for 2 hr at 37°C in duplicate wells in 96-well EIA/RIA high binding plates (Corning Inc., New York, NY) that were previously coated for 18 hr at 4°C with graded doses of bovine HA (Sigma-Aldrich) and blocked with 2% milk protein. After extensive washing with PBS/0·1% Tween-20, immobilized, biotinyated aggrecan was measured with ExtrAvidin®−Peroxidase (Sigma-Aldrich) and 3,3′,5,5′-tetramethyl benzidine/PO4/H2O2 using an EL800 plate reader (BioTek, Winooski, VT) at 450 nm. To measure the stability of this form of immobilized aggrecan, plates were further incubated for various times at 37°C and supernatants were transferred to fresh plates coated with 3 μg/ml mouse anti-bovine aggrecan monoclonal antibody, C7.1.27 Capture of released biotinylated aggrecan was measured as described above.
Preparation of bovine nasal cartilage explants
Discs (4 cm2) of articular cartilage, freshly dissected and cleaned of connective tissue were washed six or seven times in PBS supplemented with 50 U/ml nystatin (Sigma-Aldrich) and maintained at 37°C for 2 days in daily changed, serum-free media (Invitrogen) supplemented with 100 μg/ml kanamycin and nystatin in 24-well plates.
Flow cytometry
B cells (2·5 × 105) were incubated either on ice with 10 nm biotinyated aggrecan or at 37°C in wells containing immobilized aggrecan (generated by the addition of 10 nm biotinylated aggrecan to HA-coated plates as above) for various times. Cells were removed, washed with PBS/2% fetal calf serum and incubated on ice with streptavidin-APC (SA-APC, BD Pharmingen, Franklin Lakes, NJ) for 30 min and then washed. A total of 5 × 103 events were collected on a Becton Dickinson FACS Canto and analysed using facs diva software (BD, Oxford, UK).
Antigen presentation assays
Assays were performed in serum-free media in duplicate wells. In assays using aggrecan or biotinyated aggrecan, 5 × 104 B cells were incubated with 3 × 104 T-cell hybridomas for 24 hr in flat-bottomed 96-well plates containing graded doses of antigen. Alternatively, 5 × 104, or 6 × 105 B cells were incubated either in HA-coated, 96-well plates prepared with graded doses of biotinylated aggrecan, or in 24-well plates containing bovine nasal cartilage explants for various times. Following incubation with immobilized aggrecan, B cells were removed, washed and transferred to fresh 96-well plates containing 3 × 104 T-cell hybridomas for a further 24 hr. B cells that were incubated with bovine nasal cartilage were removed, washed and graded numbers were added to 5 × 104 T-cell hybridomas for 24 hr. In assays using TCR-5/4E8 T cells, 1 × 105 A20-agg B cells, that had been incubated in 96-well plates (prepared with HA and 10 nm biotinylated aggrecan) were removed, washed and co-cultured with 5 × 105 purified CD4+ T cells for 72 hr. Supernatants from replicate plates containing either immobilized biotinylated aggrecan, or bovine nasal cartilage explants were removed (following incubation at 37°C for equivalent times in the absence of B cells) and added to new plates containing appropriate numbers of fresh B and T cells. T-cell activation was measured by quantifying interleukin-2 (IL-2) production. To measure IL-2 present in assay supernatants, aliquots were transferred to fresh plates containing 3 × 104 of the IL-2-dependent T-cell line, CTLL-230 in the presence of 18·5 kBq methyl-[3H]thymidine (74 GBq/mmol; PerkinElmer, Cambridge, UK) for 24 hr as described previously.14 Radioactivity was quantified using liquid scintillation counting (Perkin Elmer, Cambridge, UK) and results are presented as mean counts per minute (cpm) ± standard error of the mean (SEM) of duplicate wells. Interferon-γ (IFN-γ) present in assay supernatants was measured using ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN) with a spectrophotometer (Molecular Devices, Sunnyvale, CA). Concentrations of IL-2 and IFN-γ in supernatants were calculated from standard curves prepared with recombinant cytokines.
Statistical analysis
Means of experimental data were compared using unpaired Student's t-test, or were compared with single baseline values using a one-sample Student's t-test where appropriate, using prism software (GraphPad Software, La Jolla, CA). P-values < 0·05 were considered significant.
Results
Aggrecan-specific B cells acquire immobilized antigen as efficiently as soluble antigen
To assess if antigen-specific B cells could acquire ECM-tethered antigens in a BCR-dependent manner, we developed a simple model of ECM, mimicking the structure of cartilage,23 in which aggrecan is immobilized by interaction with HA. The addition of biotin to soluble, deglycosylated aggrecan allowed us to detect aggrecan in this model. Control experiments showed that this modification did not affect its uptake and efficient presentation to the CD4+ T-cell hybridoma 192, specific for the aggrecan p84-103 epitope by previously generated aggrecan-specific B cells (A20-agg) when compared with unmodified aggrecan (Fig. 1a). To establish an immobilized form of aggrecan, soluble biotinylated aggrecan was incubated in HA-coated ELISA plates for 2 hr at 37°C and binding was detected by addition of ExtrAvidin®−Peroxidase. The formation of this ‘immobilized aggrecan’ was seen to be dependent on aggrecan concentration, and was HA-dependent (Fig. 1b). In addition the formation of immobilized aggrecan was relatively efficient as the levels of 10 nm biotinylated aggrecan binding detected in 2 hr in the presence of HA were similar to those following its addition to uncoated plates for 18 hr (Fig. 1b). Importantly, immobilized aggrecan prepared on HA-coated plates with up to 10 nm biotinylated aggrecan was also stable and demonstrated minimal dissociation after additional incubation periods (up to 24 hr) at 37°C (Fig. 1c).
Figure 1.
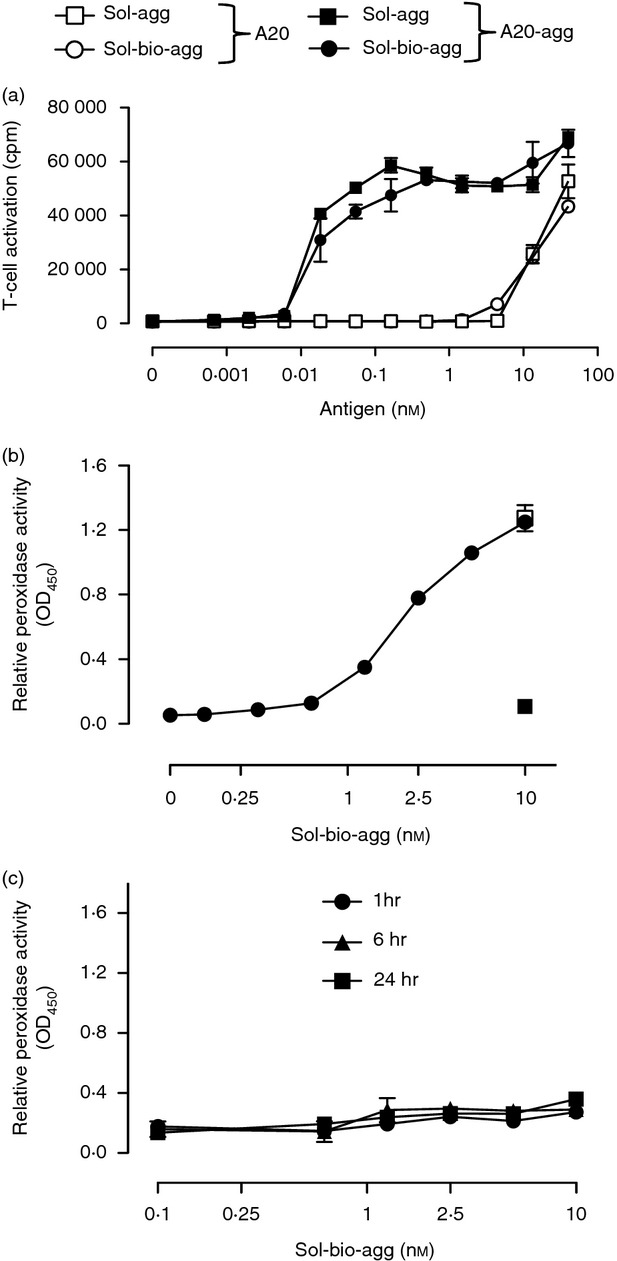
Generation of stable, immobilized aggrecan, representative of aggrecan found in cartilage extracellular matrix (ECM). (a) Biotinylation of soluble aggrecan does not affect its efficient presentation by aggrecan-specific B cells to aggrecan-specific T cells. Indicated B cells were incubated with graded doses of unmodified aggrecan (sol-agg) or biotinylated aggrecan (sol-bio-agg) and the p84-103 specific T-cell hybridoma 192 for 24 hr. Interleukin-2 (IL-2) production was determined using CTLL-2 as described in the Materials and methods. Experiment shown is representative of three. (b) Graded doses of biotinylated aggrecan were incubated for 2 hr at 37°C in hyaluronan (HA)-coated plates (•). In separate uncoated wells, 10 nm biotinylated aggrecan was also added for 2 hr at 37°C (▪) or 18 hr at 4°C (□). (c) Following the generation of immobilized aggrecan as shown in (b), plates were incubated for a further 1, 6 and 24 hr at 37°C. The levels of aggrecan dissociation were determined by transferring supernatants to new plates pre-coated with anti-aggrecan antibody C7.1.27 In both (b) and (c) biotinylated aggrecan binding was detected using ExtrAvidin®−Peroxidase and results are expressed as relative peroxidase activity [optical density at 450 nm (OD450) units (± SEM)].
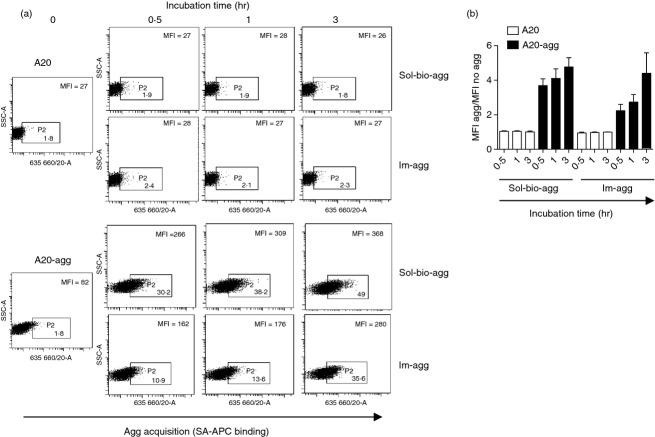
To address whether antigen-specific B cells were capable of acquiring immobilized aggrecan, we incubated A20-agg or control A20 B cells in HA-coated ELISA plates prepared with 10 nm biotinylated aggrecan as before for various times at 37°C. For comparison, we also incubated B cells with soluble biotinylated aggrecan on ice. Following incubation and washing, biotinylated aggrecan bound to the B-cell surface was labelled with SA-APC and detected by flow cytometry. A representative experiment (Fig. 2a) and a summary of three independent experiments (Fig. 2b) are shown. As expected from previous studies,27 A20-agg (but not A20) B cells bound soluble biotinylated aggrecan following incubation on ice. In addition, A20-agg (but not A20) B cells also acquired biotinylated aggrecan in a time-dependent manner following their incubation in plates containing immobilized aggrecan. Both the kinetics and amounts of biotinylated aggrecan acquired by the A20-agg B cells were similar to that bound following incubation with soluble biotinylated aggrecan.
Figure 2.
Aggrecan-specific B cells acquire immobilized aggrecan. (a) Indicated B cells were incubated either on ice with 10 nm biotinylated aggrecan (sol-bio-agg) or at 37°C in plates containing immobilized aggrecan (im-agg), established with 10 nm biotinylated aggrecan for times shown. Cells without antigen or incubation were used as controls (incubation time 0). B cells were removed, washed and incubated with streptavidin–APC (SA-APC) on ice for 30 min before flow cytometry. Aggrecan acquisition was quantified (large numbers in each panel) as mean fluorescence intensity (MFI) of all cells. (Small numbers represent % of total cells that fall within shown gates.) One experiment representative of three is shown. (b) Mean aggrecan acquisition from three independent experiments. Bars show mean MFI following incubation in the presence/absence of antigen (± SEM) for each time-point.
Aggrecan-specific B cells activate aggrecan-specific CD4+ T cells following acquisition of immobilized aggrecan
To determine whether the acquisition of aggrecan from a HA-immobilized form by specific B cells leads to presentation to T cells, A20-agg (or control A20) B cells were incubated for various time points in plates containing graded doses of immobilized aggrecan prepared as described in Fig. 1(b). B cells were then removed, washed and co-cultured for 24 hr, in the absence of additional antigen, with the p84-103-specific CD4+ T-cell hybridoma 192. A representative experiment and a summary of four independent experiments are shown in Fig. 3(a) and Fig. 3(b), respectively. A20-agg (but not A20) B cells acquired sufficient immobilized aggrecan to induce 192 T-cell activation in times as short as 30 min (Fig. 3a, left panel). Importantly, antigen acquisition and presentation by A20-agg B cells was only seen following direct contact with immobilized aggrecan. T cells cultured with fresh A20-agg B cells in the presence of supernatants removed from wells containing immobilized aggrecan that had been incubated at 37°C for up to 24 hr did not release significant amounts of IL-2 (Fig. 3a,b). Hence, antigen-specific B cells acquire immobilized aggrecan in a BCR-dependent and a contact-dependent manner.
Figure 3.
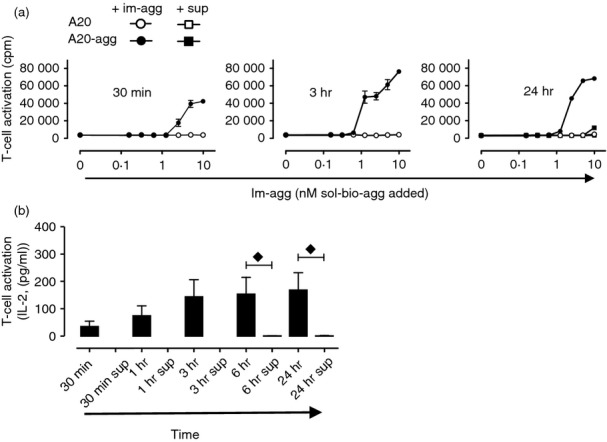
Contact-dependent acquisition of immobilized aggrecan by antigen-specific B cells leads to efficient CD4+ T-cell activation. (a) Indicated B cells were incubated in duplicate wells containing graded amounts of immobilized aggrecan (im-agg, ○,•) for the times shown, removed, washed and incubated with T-cell hybridoma 192 for 24 hr. Supernatants (sup, □, ▪) from replica wells containing graded amounts of im-agg incubated for identical times (results only shown for 24 hr; right hand panel) in the absence of B cells were transferred to new wells containing fresh B cells and 192 T cells for a further 24 hr. T-cell activation was measured by the detection of interleukin-2 (IL-2) in supernatants as described in the Materials and methods. Experiment shown is representative of four. (b) statistical analysis of results obtained from four independent experiments. Shown are the mean (± SEM) IL-2 levels (pg/ml) 24 hr after co-culture with A20-agg B cells incubated (for times shown) with im-agg (prepared with 10 nm biotinylated aggrecan) or with fresh A20-agg B cells and supernatants (sup) removed (after times shown) from B-cell-free wells containing im-agg prepared with 10 nm biotinylated aggrecan. Time-points where the mean cytokine levels produced following T-cell incubation with A20-agg B cells previously incubated with im-agg were significantly (P < 0·05) different from those following T-cell incubation with fresh A20-agg B cells and supernatants removed from wells containing im-agg (sup) (calculated using two-sample unpaired t-tests) are indicated (♦). (IL-2 in wells containing T cells, A20-agg and sup removed after 30 min, 1 hr, and 3 hr was below detectable levels.)
As both RA and PGIA are associated with the production of IFN-γ from activated CD4+ T cells,31,32 we next examined whether the acquisition of immobilized aggrecan by A20-agg B cells resulted in IFN-γ production by primary CD4+ T cells. A20-agg B cells were incubated for various times in plates containing immobilized aggrecan generated using 10 nm biotinylated aggrecan. B cells were removed, washed and co-cultured for 72 hr with splenic CD4+ T cells purified from p84-103 aggrecan-specific TCR transgenic (TCR-5/4E8) mice.29 As seen using the CD4+ T-cell hybridoma 192, A20-agg B cells acquired sufficient immobilized aggrecan in a contact-dependent manner in periods as short as 30 min to induce IL-2 release from TCR-5/4E8 CD4+ T cells (Fig. 4a). In addition, the incubation of TCR-5/4E8 CD4+ T cells with A20-agg B cells that had been incubated with immobilized aggrecan also led to the release of IFN-γ (Fig. 4b). As seen with IL-2 production, TCR-5/4E8 CD4+ T cells only produced IFN-γ following incubation with A20-agg that were previously incubated with immobilized aggrecan but not following incubation with fresh A20-agg B cells and supernatants taken from wells containing immobilized aggrecan (Fig. 4b). Hence, aggrecan-specific B cells acquire HA-tethered aggrecan leading to the activation and differentiation of aggrecan-specific CD4+ T cells.
Figure 4.
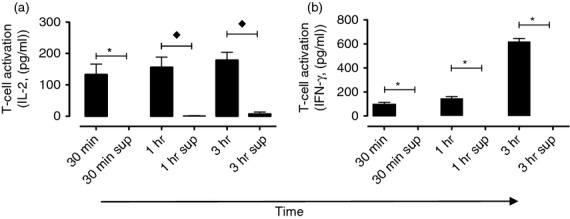
Contact-dependent, immobilized aggrecan acquisition by antigen-specific B cells leads to efficient CD4+ T-cell differentiation and interferon-γ (IFN-γ) production. T-cell receptor (TCR)-5/4E8 CD4+ T cells were cultured either with A20-agg B cells that had previously been incubated (for times shown) in wells containing immobilized aggrecan (im-agg; established with 10 nm biotinylated aggrecan) for 72 hr. In addition, TCR-5/4E8 CD4+ T cells were also cultured (72 hr) with fresh A20-agg B cells and supernatants (sup) that were removed (after times shown) from wells containing im-agg but lacking B cells. Levels of (a) interleukin-2 (IL-2) or (b) IFN-γ present in assay supernatants were determined as described in the Materials and methods. Mean cytokine levels (± SEM) are shown from each time-point from three independent experiments. Time-points where the mean cytokine levels produced following T-cell incubation with A20-agg B cells previously incubated with im-agg were significantly (P < 0·05) different from those following T-cell incubation with fresh A20-agg B cells and supernatants removed from im-agg (sup) (calculated using one-sample (⋆), or two-sample (♦) unpaired t-tests) are indicated. (IL-2 in wells containing T cells, A20-agg and sup removed from im-agg after 30 min was below detectable levels. Similarly, IFN-γ in wells containing T cells, A20-agg and sup removed from im-agg after 30 min, 1 hr and 3 hr was below detectable levels).
Aggrecan-specific B cells acquire aggrecan from intact cartilage for presentation to CD4+ T cells
To test whether aggrecan-specific B cells acquire and present aggrecan from physiological cartilage ECM, we used a modification of an established cartilage explant model.33 A20-agg (or A20) B cells were added to extensively washed, ex vivo isolated cartilage explants for various times. B cells were then removed, washed and graded numbers were re-cultured with 192 T-cell hybridomas for 24 hr in the absence of additional antigen. As performed previously, control experiments included 192 T-cell hybridomas incubated with fresh B cells and supernatants removed from wells containing cartilage explants at various time-points. Figure 5(a) shows that A20-agg (but not A20) B cells efficiently acquired and presented aggrecan from cartilage explants with kinetics similar to that seen when incubated with immobilized aggrecan. Indeed, A20-agg B cells incubated with cartilage explants for times as short as 1 min were capable of activating 192 T-cell hybridomas (Fig. 5a). Although limited presentation of aggrecan by A20-agg B cells resulted from the capture of aggrecan released into the supernatants following 4 hr of incubation (Fig. 5b, ‘4 hr sup’), A20-agg (but not A20) B cells acquired sufficient aggrecan in a contact-dependent manner for subsequent T-cell presentation in the shorter time periods measured (Fig. 5b).
Figure 5.
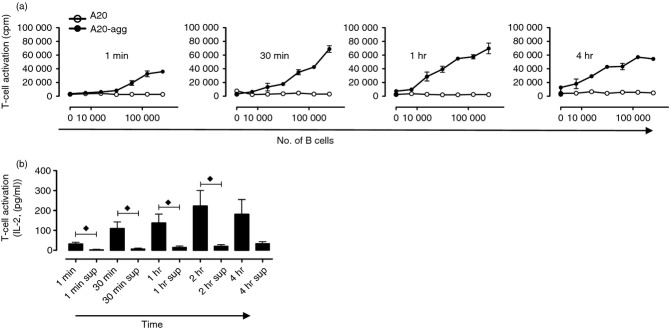
Aggrecan-specifc B cells acquire cartilage-derived aggrecan in a contact-dependent manner leading to CD4+ T-cell activation. (a) Indicated B cells were incubated in wells containing bovine nasal cartilage (BNC) explants for the times shown, removed, washed and graded numbers were incubated with 192 T-cell hybridomas for 24 hr. T-cell activation was measured by the detection of interleukin-2 (IL-2) in supernatants as described in the Materials and methods; experiment shown is representative of five. (b) Statistical analysis of results obtained from five independent experiments. The mean (± SEM) of IL-2 levels (pg/ml) are shown for 24 hr after 192 T-cell hybridomas were co-cultured with 1·25 × 105 A20-agg B cells that were previously incubated (for times shown) with cartilage or with fresh A20-agg B cells and supernatants (sup) removed (after times shown) from B cell-free wells containing cartilage. The IL-2 levels were measured as described in the Materials and methods. Time-points where mean IL-2 levels following incubation + BNC were significantly different (P < 0·05) from those following incubation with sup (using two-sample unpaired t-tests) are indicated (♦).
Discussion
Studies using either the PGIA8,34 or other animal models of autoimmune disorders6,7, as well as recent findings from RA patients,21 suggest that B cells perform an essential antigen-presenting function in disease initiation and progression. However, the precise role that sets B cells apart from other APC remains unknown. In addition to the uptake of soluble antigen, it is now appreciated that antigen-specific B cells also extract antigens that are either tethered or integral to the surface of other cells.14,35 Here we demonstrate for the first time that antigen-specific B cells are also capable of acquiring integral ECM antigens in a contact-dependent manner. In particular, we demonstrate that antigen-specific B cells acquire the cartilage ECM proteoglycan aggrecan, either immobilized in a model form, or directly from intact cartilage itself. We also demonstrate that aggrecan acquired from ECM in this manner leads to the activation and differentiation of aggrecan-specific CD4+ T cells and that this process is extremely efficient. As it is known that aggrecan is released from ECM,24 we have excluded this source of antigen in our experiments by demonstrating that supernatants removed from wells containing either immobilized aggrecan or cartilage explants contain insufficient aggrecan for presentation by A20-agg B cells. Hence, BCR-mediated acquisition of aggrecan from ECM is contact-dependent, similar to that seen previously with the extraction of membrane-associated antigens.14,35
Kinetic analysis demonstrated that the acquisition of aggrecan from ECM occurs in times as short as 1 min (Fig. 5), which are directly comparable to the kinetics we and others have measured previously for the acquisition of antigens from cell surfaces.14,35 BCR-mediated recognition of antigens on the surface of target cells triggers the formation of a dynamic synapse between the two cell types.13 Following BCR/antigen ligation, the B-cell plasma membrane spreads over the target cell followed by a contraction phase in which antigen is ‘collected’ into a central cluster.36 This process involves remodelling of the B-cell cytoskeleton by members of the Ezrin-Radixin-Moesin family of actin-binding proteins,37 as well as interactions between the integrins leucocyte function-associated antigen 1 (LFA-1) and very late antigen-4 (VLA-4) and their respective ligands intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) expressed on the target cell.38,39 Although LFA-1/ICAM-1 interactions form a peripheral molecular ring surrounding a central BCR/antigen cluster that functions largely to stabilize the synapse, reducing the threshold for antigen required on the target cell, they are not essential for synapse formation.38 In contrast, VLA-4/VCAM-1 interactions appear to occur earlier in synapse formation and serve to additionally amplify BCR-mediated B-cell activation.39 Interestingly VCAM-1 expression is up-regulated in synovial fibroblasts from RA patients and the VLA-4 alternative ligand, fibronectin, is present in cartilage ECM40 which could both facilitate B-cell antigen presentation in the inflamed joint. It has also been demonstrated that the spreading of the B-cell plasma membrane over a surface arrayed with BCR-reactive antigen facilitates efficient presentation by low-affinity BCR.36 It is therefore possible that BCR-mediated acquisition of immobilized antigens from ECM, resulting from a similar process, also improves the efficiency of presentation by B cells expressing lower affinity receptors.
For membrane-associated antigens, the mechanisms involved in BCR-mediated extraction and the subsequent delivery into the B-cell endocytic pathways for enzymatic processing and association with MHC class II molecules are not clearly defined. A recent report from Natkanski et al.41 using atomic-force microscopy to measure antigen uptake from immobilized plasma membrane sheets, demonstrated BCR-mediated ‘pinching off’ of antigen along with the adjacent phospholipid bilayer. In contrast, others have reported that the release of B-cell lysosomal contents into the target/B-cell space was essential for antigen extraction.42 Although the nature of BCR-mediated antigen acquisition from ECM has yet to be addressed, it will possibly share some of the features seen with membrane-associated antigens.
To maintain functional cartilage ECM, the biosynthesis of multiple components, including aggrecan, is carefully regulated together with members of the families of catabolic enzymes (including matrix metalloproteinases).43 During inflammatory joint disease, however, this balance is lost as a result of cytokine-driven increases in catabolic enzymatic activity.44 This results in preferential release of C-terminal aggrecan fragments from the matrix into the synovial fluid with remnants of the N-terminal G1 and G2 domains remaining attached to the ECM.24 As most of the reported arthritogenic CD4+ T-cell epitopes of aggrecan are located within the N-terminal G1 domain,25,45 it has not been clear how APC gain access to these ECM-anchored N-terminal regions to generate the T-cell epitopes of this candidate autoantigen. We now show for the first time that antigen-specific B cells efficiently directly acquire the RA candidate autoantigen aggrecan from cartilage explants in a contact-dependent manner. Our experiments also show that this acquisition results in the presentation of the major arthritogenic T-cell epitope of aggrecan to CD4+ T cells leading to their differentiation into cytokine-producing effector cells. As this epitope is located with the N-terminal G1 domain, and we show minimal presentation resulting from released aggrecan, our findings are consistent with previous studies demonstrating the presence of C-terminal aggrecan fragments within synovial fluid with N-terminal fragments predominantly remaining ECM-associated. Our findings therefore offer an explanation for the mechanism underlying B-cell-mediated presentation of the T-cell epitopes from this candidate RA autoantigen and implicate the acquisition of ECM antigens as a defining feature of B-cell APC function essential for the development of RA. As ECM is a unifying feature of most tissues, our findings therefore have significant implications for the role of B-cell-mediated antigen acquisition and presentation in other autoimmune conditions.
Acknowledgments
We thank T. Williamson for technical support with the bovine nasal cartilage preparations, Dominic W. Hine for help and advice with intracellular cytokine staining and Bram Margry for additional experiments using the TCR-5/4E8 transgenic mice. This work was supported by grants from the JGW Patterson Foundation and the Newcastle Healthcare Charity and Newcastle upon Tyne Hospitals NHS Charity. CLW was supported by an Arthritis Research UK PhD studentship. AF was supported by a Newcastle University PhD studentship.
Glossary
Abbreviations
- APC
antigen-presenting cell
- BCR
B-cell receptor
- BNC
bovine nasal cartilage
- ECM
extracellular matrix
- HA
hyaluronan
- ICAM-1
intercellular adhesion molecule 1
- IFN-γ
interferon-γ
- IL-2
interleukin-2
- LFA-1
leucocyte function-associated antigen 1
- PGIA
proteoglycan-induced arthritis
- RA
rheumatoid arthritis
- TCR
T-cell receptor
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late antigen-4
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- 1.Ziegler K, Unanue ER. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981;127:1869–75. [PubMed] [Google Scholar]
- 2.Ueno H, Schmitt N, Klechevsky E, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–59. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 5.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891–9. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong FS, Wen L, Tang M, et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–7. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–8. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 9.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 10.Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-γ production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–12. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel KJ, Neuberger MS. Antigen presentation by the B cell antigen receptor is driven by the α/β sheath and occurs independently of its cytoplasmic tyrosines. Cell. 1993;74:939–46. doi: 10.1016/0092-8674(93)90473-4. [DOI] [PubMed] [Google Scholar]
- 12.Knight AM, Lucocq JM, Prescott AR, Ponnambalam S, Watts C. Antigen endocytosis and presentation mediated by human membrane IgG1 in the absence of the Igα/Igβ dimer. EMBO J. 1997;16:3842–50. doi: 10.1093/emboj/16.13.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 14.Ciechomska M, Lennard TW, Kirby JA, Knight AM. B lymphocytes acquire and present intracellular antigens that have relocated to the surface of apoptotic target cells. Eur J Immunol. 2011;41:1850–61. doi: 10.1002/eji.201141472. [DOI] [PubMed] [Google Scholar]
- 15.Lin RH, Mamula MJ, Hardin JA, Janeway CA., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991;173:1433–9. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–9. [PubMed] [Google Scholar]
- 17.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 18.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–7. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 19.Gavanescu I, Benoist C, Mathis D. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: a therapeutic approach for APECED patients. Proc Natl Acad Sci U S A. 2008;105:13009–14. doi: 10.1073/pnas.0806874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl 5):v3–11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 21.Mauri C, Ehrenstein MR. Cells of the synovium in rheumatoid arthritis. B cells. Arthritis Res Ther. 2007;9:205–10. doi: 10.1186/ar2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everts V, Buttle DJ. Methods in studying ECM degradation. Methods. 2008;45:86–92. doi: 10.1016/j.ymeth.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Cheung SC, Itano N, Kimata K, Yamada Y. Identification of hyaluronan-binding domains of aggrecan. J Biol Chem. 1997;272:28057–65. doi: 10.1074/jbc.272.44.28057. [DOI] [PubMed] [Google Scholar]
- 24.Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–22. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- 25.Leroux JY, Guerassimov A, Cartman A, et al. Immunity to the G1 globular domain of the cartilage proteoglycan aggrecan can induce inflammatory erosive polyarthritis and spondylitis in BALB/c mice but immunity to G1 is inhibited by covalently bound keratan sulfate in vitro and in vivo. J Clin Invest. 1996;97:621–32. doi: 10.1172/JCI118458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glant TT, Buzas EI, Finnegan A, Negroiu G, Cs-Szabo G, Mikecz K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812–9. [PubMed] [Google Scholar]
- 27.Wilson CL, Hine DW, Pradipta A, et al. Presentation of the candidate rheumatoid arthritis autoantigen aggrecan by antigen-specific B cells induces enhanced CD4+ T helper type 1 subset differentiation. Immunology. 2012;135:344–54. doi: 10.1111/j.1365-2567.2011.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–54. [PubMed] [Google Scholar]
- 29.Berlo SE, van Kooten PJ, Ten Brink CB, et al. Naive transgenic T cells expressing cartilage proteoglycan-specific TCR induce arthritis upon in vivo activation. J Autoimmun. 2005;25:172–80. doi: 10.1016/j.jaut.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–6. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 31.Hollo K, Glant TT, Garzo M, Finnegan A, Mikecz K, Buzas E. Complex pattern of Th1 and Th2 activation with a preferential increase of autoreactive Th1 cells in BALB/c mice with proteoglycan (aggrecan)-induced arthritis. Clin Exp Immunol. 2000;120:167–73. doi: 10.1046/j.1365-2249.2000.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doodes PD, Cao Y, Hamel KM, et al. Development of proteoglycan-induced arthritis is independent of IL-17. J Immunol. 2008;181:329–37. doi: 10.4049/jimmunol.181.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cawston TE, Ellis AJ, Humm G, Lean E, Ward D, Curry V. Interleukin-1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Commun. 1995;215:377–85. doi: 10.1006/bbrc.1995.2476. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–16. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 35.Batista FD, Neuberger MS. B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 2000;19:513–20. doi: 10.1093/emboj/19.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–41. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 37.Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med. 2011;208:1055–68. doi: 10.1084/jem.20101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–99. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 39.Carrasco YR, Batista FD. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 2006;25:889–99. doi: 10.1038/sj.emboj.7600944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales-Ducret J, Wayner E, Elices MJ, Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. α4/β1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992;149:1424–31. [PubMed] [Google Scholar]
- 41.Natkanski E, Lee WY, Mistry B, Casal A, Molloy JE, Tolar P. B cells use mechanical energy to discriminate antigen affinities. Science. 2013;340:1587–90. doi: 10.1126/science.1237572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuseff MI, Reversat A, Lankar D, et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity. 2011;35:361–74. doi: 10.1016/j.immuni.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Rowan AD, Young DA. Collagenase gene regulation by pro-inflammatory cytokines in cartilage. Front Biosci. 2007;12:536–50. doi: 10.2741/2080. [DOI] [PubMed] [Google Scholar]
- 45.Buzas EI, Vegvari A, Murad YM, Finnegan A, Mikecz K, Glant TT. T-cell recognition of differentially tolerated epitopes of cartilage proteoglycan aggrecan in arthritis. Cell Immunol. 2005;235:98–108. doi: 10.1016/j.cellimm.2004.08.006. [DOI] [PubMed] [Google Scholar]