Abstract
Hypoxia is a fundamental stimulus that impacts cells, tissues, organs, and physiological systems. The discovery of hypoxia-inducible factor-1 (HIF-1) and subsequent identification of other members of the HIF family of transcriptional activators has provided insight into the molecular underpinnings of oxygen homeostasis. This review focuses on the mechanisms of HIF activation and their roles in physiological and pathophysiological responses to hypoxia, with an emphasis on the cardiorespiratory systems. HIFs are heterodimers comprised of an O2-regulated HIF-1α or HIF-2α subunit and a constitutively expressed HIF-1β subunit. Induction of HIF activity under conditions of reduced O2 availability requires stabilization of HIF-1α and HIF-2α due to reduced prolyl hydroxylation, dimerization with HIF-1β, and interaction with coactivators due to decreased asparaginyl hydroxylation. Stimuli other than hypoxia, such as nitric oxide and reactive oxygen species, can also activate HIFs. HIF-1 and HIF-2 are essential for acute O2 sensing by the carotid body, and their coordinated transcriptional activation is critical for physiological adaptations to chronic hypoxia including erythropoiesis, vascularization, metabolic reprogramming, and ventilatory acclimatization. In contrast, intermittent hypoxia, which occurs in association with sleep-disordered breathing, results in an imbalance between HIF-1α and HIF-2α that causes oxidative stress, leading to cardiorespiratory pathology.
I. INTRODUCTION
In this review, we summarize the role of the hypoxia-inducible factors HIF-1 and HIF-2 in modulating gene transcription as a function of O2 availability. Due to space limitations, this work cannot encompass the entire body of exponentially increasing data regarding the important role of oxygen homeostasis in development, physiology, and disease. Here we describe several physiological (adaptive) and pathophysiological (maladaptive) cellular and systemic responses to changes in O2 availability, with a particular focus on the circulatory and respiratory systems. However, as with any problem in physiology, we must start by defining the stimulus and response.
A. The Stimulus: What Is Hypoxia?
We will define hypoxia as a reduction in O2 availability in one state or condition compared with another; as such, it is a highly relative term. Most commonly, it is used to compare different spatial or temporal conditions. Hypoxia may 1) be restricted to certain cells within a tissue, 2) be restricted to certain tissue within a specific organ, or 3) involve the entire organism. For example, 1) within the lobular architecture of the liver of a healthy human or other mammal, hepatocytes surrounding the central vein are exposed to lower oxygen concentrations than those surrounding the portal triad; 2) when the left coronary artery is occluded, cardiac tissue that is normally perfused by this artery rapidly becomes hypoxic; and 3) severe blood loss results in systemic hypoxia due to reduced O2-carrying capacity.
The duration of hypoxia can be acute (ranging from fractions of a second to minutes) or chronic (hours to days). At the molecular level, acute hypoxia induces rapid but short-lived responses that are often due to the modification of existing proteins, whereas chronic hypoxia induces delayed but durable responses that require changes in mRNA and protein expression.
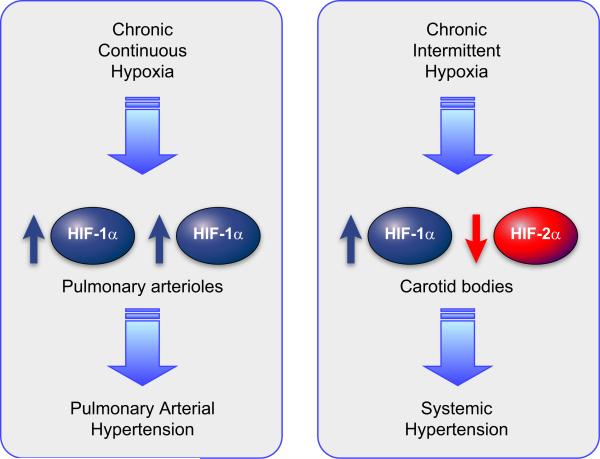
Hypoxia can be continuous or intermittent; in the latter case, oxygen concentrations alternate between low and baseline levels. Repeated episodes of hypoxia and reoxygenation appear to trigger the unique pathophysiology that is associated with intermittent hypoxia (IH) compared with continuous hypoxia (265). For example, exposure to chronic continuous hypoxia results in the development of pulmonary hypertension, whereas exposure to chronic IH results in the development of systemic hypertension.
Hypoxia is often contrasted with “normoxia,” a term that refers to the “normal” O2 concentration. There is danger inherent in the use of the term normoxia to describe the standard conditions for culturing cells ex vivo, i.e., 95% air and 5% CO2. Air is 21% O2 (corresponding to a Po2 of 147 mmHg at sea level) and 95% air therefore represents 20% O2 (140 mmHg), which is a higher concentration than that experienced by virtually any cell in the human body. The Po2 in arterial and venous blood is ~100 mmHg (~14% O2) and 40 mmHg (~6% O2), respectively. There is tremendous variation in Po2 both within and between organs. For example, microelectrode measurements of Po2 in mouse spleen ranged from 4 to 34 mmHg (37). Thus what is considered “normal” in tissue culture experiments is decidedly not normal in vivo. Exposure of cells to nonphysiological O2 concentrations (i.e., 20% O2) results in alterations in signal transduction pathways that may change the phenotype of the cells and their responses to other stimuli (81, 284). Referring to cells cultured at 20% O2 as nonhypoxic is a much more appropriate designation.
In tissue culture, hypoxia is clearly a relative term. HT22 cells, which are derived from cortical neurons that are exposed normally to ~5% O2 (~35 mmHg) in vivo, are typically cultured ex vivo in the presence of 20% O2 (140 mmHg). When these cells are shifted from 20% to 5% O2, they signal hypoxia by activating HIF-1; remarkably, if the cells are cultured for several weeks at 30% O2 (210 mmHg) and then shifted to 20% O2, they also signal hypoxia (154). Thus cultured cells have remarkable plasticity in establishing a “normoxic” set point.
It should be noted that hypoxia often occurs in vivo in the context of ischemia, which refers to a state of inadequate tissue perfusion. However, in addition to reduced O2 availability (i.e., hypoxia), ischemic tissue is also characterized by reduced energy substrates and increased tissue metabolites, including CO2, H+, K+, and lactate among others. Remarkably, the physiological response to hypoxia is sufficient to increase perfusion, thereby correcting many, if not all, of the metabolic abnormalities associated with ischemia.
Finally, many investigators who use chemical compounds (such as iron chelators and α-ketoglutarate antagonists) to induce HIF activity regrettably refer to such agents and the resulting condition of the cells as “hypoxia mimetics” and “chemical hypoxia,” respectively. These are inappropriate designations, since these agents do not reduce O2 availability nor do they induce the complete range of physiological changes that occur with hypoxia. As with use of the term normoxia in reference to cultured cells, these are inaccurate terms that should be purged from the scientific lexicon.
B. The Response: Adaptive, Failed, or Maladaptive?
Hypoxia is a fundamental physiological stimulus that evokes adaptive (homeostatic) responses. Perhaps one of the most important examples of a homeostatic response to hypoxia (from the point of view of evolutionary selection) is the erythropoietic response to hemorrhage. Blood loss leads to systemic hypoxia, due to reduced O2-carrying capacity; in response, increased erythropoiesis restores blood O2-carrying capacity, thereby correcting the hypoxia. Ascent to high altitude results in the same physiological stimulus (systemic hypoxia) but due to a different underlying cause (reduced atmospheric O2). At very high altitude (>3,000 m), erythropoiesis represents a failed response because markedly increased erythroid mass leads to increased blood viscosity, which impairs capillary blood flow, thereby negating any increase in O2 delivery that might be afforded by higher hemoglobin levels. In the case of ischemia, an important physiological response to local tissue hypoxia is angiogenesis, the sprouting of new capillaries from existing vessels. However, vascular adaptation to ischemia is impaired by aging and diabetes, and this failed response plays a critical role in the pathogenesis of coronary and peripheral arterial diseases.
In contrast to the aforementioned examples of continuous hypoxia, it can be argued that intermittent hypoxia almost always represents a pathological stimulus that evokes maladaptive responses. Natural selection against these responses has not occurred due to the recent origin (in evolutionary terms) of the underlying pathology, which is, most commonly, obesity-related transient upper airway obstruction during sleep.
II. HYPOXIA-INDUCIBLE FACTORS: MASTER REGULATORS OF OXYGEN HOMEOSTASIS
The most well-characterized response to hypoxia is the transcriptional regulation of gene expression that is mediated by HIF-1 and HIF-2. Other transcription factors have been implicated in the transcription of certain genes within particular cell types under hypoxic conditions (for illustrative examples, see TABLE 1), although in most cases the underlying mechanisms are not well delineated and the actual inducing stimulus may not be hypoxia, but rather one of its sequelae. In contrast, HIF-1 functions as a global master regulator that is induced by hypoxia in all nucleated cells within every metazoan species that has been analyzed, whereas HIF-2 is found only in vertebrate species, and although it is expressed in many tissues during embryonic development, it has a more restricted pattern of expression in cells of the adult (87, 109, 352). Furthermore, the molecular mechanisms by which changes in O2 concentration are transduced to the nucleus as changes in the activity of HIF-1 have been determined in remarkable detail. Hence, HIFs serve as the paradigm for understanding the molecular physiology of oxygen homeostasis.
Table 1.
Hypoxia-inducible gene expression mediated by factors other than HIF-1 or HIF-2
| Factor | Target Gene(s) | Tissue/Cells | Reference Nos. |
|---|---|---|---|
| AP-1 | Heme oxygenase 1 | Pulmonary artery ECs | 110 |
| Tyrosine hydroxylase | PC-12 cells | 243 | |
| Interleukin-8 (IL-8) | Ovarian carcinoma cells | 358 | |
| ATF-4 | Carbonic anhydrase 9 | Mouse embryo fibroblasts | 337 |
| LAMP3 | Human tumor cell lines | 236 | |
| CREB | Amphiregulin | T84 intestinal epithelial cells | 244 |
| IL-8, MHC-II, TNF-α | T84 intestinal epithelial cells | 327 | |
| EGR-1 | Tissue factor, ICAM-1 | Vasculature and mononuclear cells | 361-363 |
| IL-1 β, IP-10, MIP-2, | |||
| MCP-1, PAI-1, RANTES | |||
| VEGF | |||
| EGFR | Human osteosarcoma cells | 242 | |
| HMG-I(Y) | COX-2 | Human umbilical vein ECs | 140 |
| NF-IL6 | IL-6 | Vascular ECs | 364 |
| Cardiac myocytes | 218 | ||
| TNF receptor 2 | NIH 3T3 cells | 112 | |
| NF-kB | BCL-X | Brain | 98 |
| TNF-α | J774.1 macrophage cells | 44 | |
| COX-2 | Human umbilical vein ECs | 289 | |
| IL-6 | Cardiac myocytes | 218 | |
| IL-8 | Ovarian carcinoma cells | 358 | |
| IL-8, MMP-9, VEGF | Prostate cancer cells | 126 | |
| PURα | CD11b, CD11c | U937 cells | 163 |
| Sp1 | COX-2 | Human umbilical vein ECs | 359 |
| Sp1/Sp3 | PKM, β-enolase | C2C12 myocytes | 72 |
| Unknown | GADD153, p27 | Mouse embryonic stem cells | 40 |
| IAP-2 | Mouse embryonic stem cells | 74 | |
| Ornithine decarboxylase | Mouse embryonic stem cells | 135 | |
| p27 | Mouse embryo fibroblasts | 96 | |
| VEGF | Human colon cancer cell lines | 231 |
See text for definitions.
A. Erythropoietin Expression and the Discovery of HIF-1
Erythropoietin (EPO) is the glycoprotein hormone that regulates mammalian erythrocyte production and, as a result, tissue O2 delivery (138). EPO mRNA levels were found to increase several hundredfold in liver and kidney tissue of rodents exposed to reduced atmospheric O2 concentrations (75, 290, 291) or anemia (16, 21, 168). Human EPO mRNA levels showed similar increases in liver and kidney of mice transgenic for the human EPO gene (167, 297, 299, 302). Hypoxia also induced EPO mRNA expression in Hep3B human hepatoma cells (99, 101), which indicated that the same cell type can sense hypoxia and respond by increasing EPO mRNA levels. The 50-fold increase in steady-state EPO mRNA in hypoxic Hep3B cells required new protein synthesis and was accounted for by an ~10-fold increase in the rate of transcription, with the remaining increase due to posttranscriptional mechanisms (99, 100). Nuclear extracts prepared from hypoxic Hep3B cells supported a higher level of EPO gene transcription in vitro than extracts from nonhypoxic cells (57).
Investigation of the cis-acting DNA sequences required for increased gene transcription in response to hypoxia revealed the presence of a hypoxia response element (HRE) in the 3′-flanking region of the human and mouse genes encoding EPO (12, 270, 300). Presence of the HRE in cis resulted in transactivation of a heterologous reporter gene under hypoxic conditions (1% O2, 7 mmHg). The HRE in the human EPO gene was localized to a 33-bp DNA sequence, and an electrophoretic mobility-shift assay (EMSA), using an oligonucleotide probe consisting of the first 18 bp of the HRE (5′-GCCCTACGTGCTGTCTCA-3′), identified a nuclear factor that was present in cells incubated at 1% O2 but absent in cells incubated at 20% O2 (140 mmHg) and was therefore designated HIF-1 (303). A 3-bp mutation in the EMSA probe (CGT to AAA) disrupted the binding of HIF-1 and eliminated hypoxia-inducible transcription mediated by the HRE (303). The same experimental approach was used to identify HIF-1 binding sites in the genes encoding vascular endothelial growth factor (VEGF) and the glycolytic enzyme enolase 1, which contained the core sequence 5′-ACGTG-3′ or 5′-GCGTG-3′ and CGT to AAA substitutions resulted in loss of HIF-1 binding and HRE function (90, 298). Subsequently, this same paradigm has been applied to the characterization of dozens of additional hypoxia-inducible genes over the last 15 years.
B. HIF Structure and Function
1. HIF-1
The HIF-1 protein was purified from 100 liters of HeLa cells grown in suspension culture by DNA affinity chromatography based on its ability to bind to the wild type (WT) but not to the mutant EMSA oligonucleotide; the purified protein consisted of two subunits, which were designated HIF-1α and HIF-1β (344). Based on tryptic peptide sequence data, degenerate oligonucleotides were designed to screen a Hep3B cDNA library and isolate cDNA clones encoding HIF-1α and HIF-1β (343). The cDNA sequences revealed that HIF-1α was a novel 826-amino acid protein, whereas HIF-1β was identical to the 789-amino acid aryl hydrocarbon receptor (AHR) nuclear translocator (ARNT) protein, which was known to heterodimerize with AHR in the presence of aryl hydrocarbons such as dioxin (116). Thus, whereas ARNT/HIF-1β is a subunit that is common to several heterodimeric transcription factors, HIF-1α is the unique and defining subunit of HIF-1.
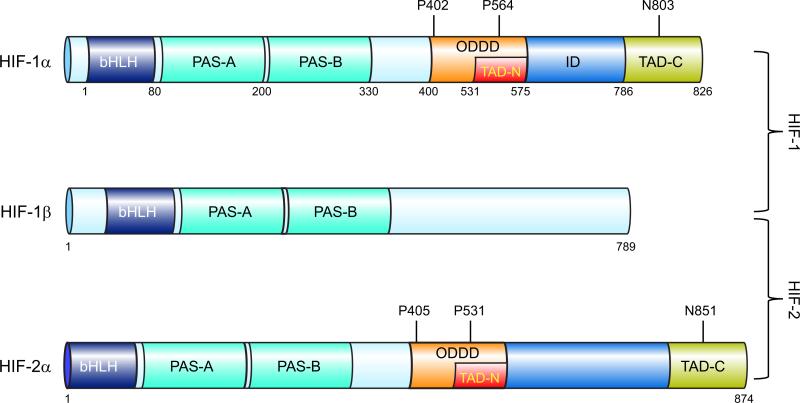
The NH2-terminal half of HIF-1α and HIF-1β consists of bHLH (basic helix-loop-helix) and PAS (Per-ARNT-Sim homology) domains (FIGURE 1) that are required for heterodimerization and DNA binding (142). The COOH-terminal half of HIF-1α (residues 531–826) contains two transactivation domains (TADs), which are designated TAD-N (amino acid residues 531–575) and TAD-C (residues 786–826) separated by an inhibitory domain (residues 576–785) (FIGURE 1). Fusion proteins consisting of the DNA binding domain of the yeast Gal4 transcription factor fused to the entire COOH-terminal half of HIF-1α, TAD-N, or TAD-C mediated hypoxia-inducible transcription of a reporter gene containing Gal4 binding sites (144, 269).
FIGURE 1.
Domain structure of hypoxia-inducible factor (HIF) subunits. The following domains are shown: basic helix-loop-helix domain (bHLH), Per-Arnt-Sim homology domain (PAS), O2-dependent degradation domain (ODDD), NH2- and COOH-terminal transactivation domains (TAD-N and TAD-C).
Analysis of HIF-1α and HIF-1β protein levels in HeLa cells revealed that HIF-1α levels increased dramatically at O2 concentrations below 6% (42 mmHg), with half-maximal induction at ~1.5% (10.5 mmHg) and maximal induction at 0.5% (3.5 mmHg) and rapid decay upon reoxygenation to 20% O2 (140 mmHg) with a half-life of <5 min (143, 344). The short half-life of HIF-1α under nonhypoxic and posthypoxic conditions is due to rapid ubiquitination and proteasomal degradation (125, 149, 285). HIF-1α residues 400–600 were required for this effect (125), and this region was designated the oxygen-dependent degradation domain (ODDD; FIGURE 1). Analysis of nuclear extracts revealed modestly increased levels of HIF-1β under hypoxic conditions (143, 344), whereas analysis of whole cell lysates did not, because the presence of HIF-1α increases the retention of HIF-1β in the nuclei of hypoxic cells (48). HIF-1-dependent gene transcription was induced by forced expression of HIF-1α but not HIF-1β, demonstrating that HIF-1α levels were limiting (142, 298).
2. Alternative splicing of HIF-1 primary RNA transcripts
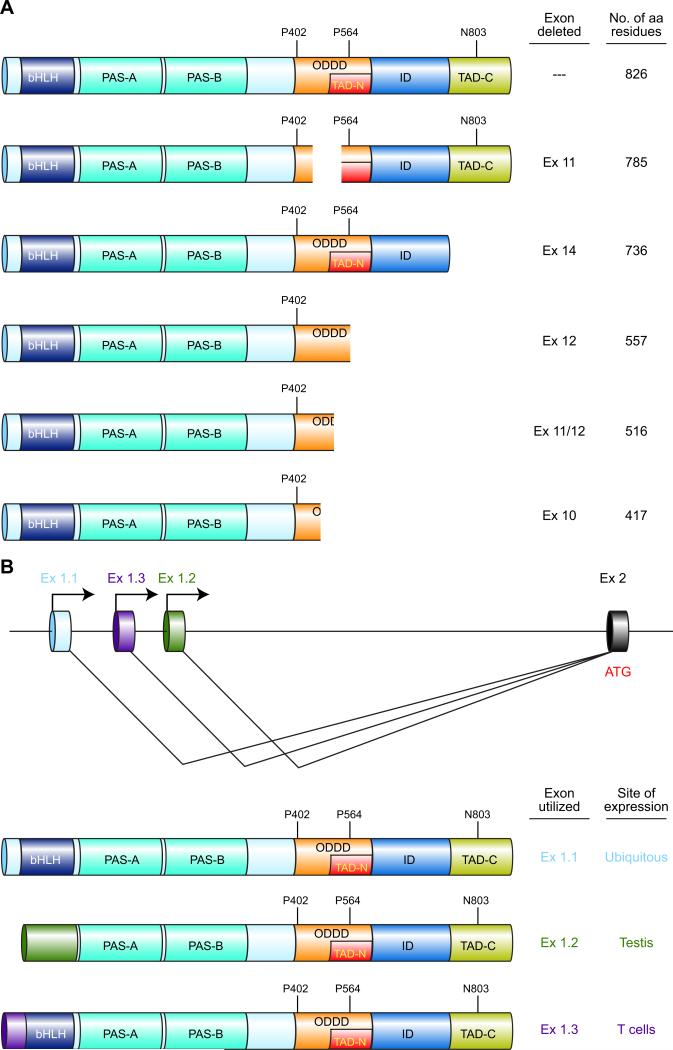
HIF-1α is encoded by the HIF1A gene (Unigene accession no. Hs.597216), which was mapped to the long arm of human chromosome 14(301). Comparison of the original cDNA sequence (GenBank accession no. U22431) encoding the 826-amino acid HIF-1α polypeptide described above (343) with the genomic DNA sequence revealed that the mature mRNA was encoded by 15 exons (136). Subsequently, alternative mRNA isoforms were identified in which exon 10, 11, 12, or 14 was spliced out of the variant mRNA, resulting in shorter HIF-1α polypeptides (FIGURE 2A) with altered biological properties (50–52, 102, 186). The involvement of these variant polypeptides in physiological and pathological responses to hypoxia has not been well studied to date.
FIGURE 2.
Multiple HIF-1α isoforms are generated as a result of alternative splicing and alternative promoter utilization. A: alternative splicing of the primary RNA transcript generates mRNAs encoding six different isoforms with the indicated domain composition. B: alternative promoter utilization results in three alternative first exons (Ex 1.1, 1.2, and 1.3) and alternative NH2-terminal amino acid sequences with the translation initiation codon (ATG) present either in the first exon (Ex 1.1, 1.3) or second exon (Ex 1.2).
In addition to the promoter that is utilized in all tissues to transcribe the primary RNA from which the HIF-1α iso-forms described above (which differ at their COOH terminus; FIGURE 2A) are generated by alternative splicing, there are two alternative promoters, located in intron 1 of the HIF1A gene, that are utilized in a tissue-specific manner to generate isoforms of HIF-1α that differ from the ubiquitous isoform at their NH2 terminus (FIGURE 2B). Utilization of Exon 1.1 generates the ubiquitous isoform, whereas utilization of Exon 1.3 in activated T lymphocytes generates a variant isoform in which the NH2-terminal residues prior to the bHLH domain are different from those encoded by Exon 1.1 (204). In HIF-1α (Ex1.3) the first 12 amino acids of HIF-1α (Ex1.1) are replaced by a novel 36-amino acid sequence. Remarkably, HIF-1α (Ex1.3) represses transcription of an HRE-dependent reporter gene (204), suggesting that HIF-1α (Ex1.3) competes with HIF-1α (Ex1.1) for dimerization with HIF-1β, but the resulting heterodimers may be incapable of binding to DNA due to the different residues immediate NH2-terminal to the basic domain that binds DNA. Genetic deletion of the T-cell-specific isoform in mice results in enhanced T-cell function (203), suggesting that the expression of HIF-1α (Ex1.3) is an important feedback mechanism to downregulate T-cell activation. In testis, Exon 1.2 is utilized and results in a protein that lacks the bHLH domain and may therefore inhibit HIF-1 activity, although its localization to the flagellum of spermatozoa (214) suggests that it is functioning as a structural protein.
Alternative splicing of the primary mRNA transcribed from the ARNT gene results in isoforms of 774 and 789 amino acid residues (116). Both isoforms are capable of dimerizing with HIF-1α and forming transcriptionally active heterodimers. Physiologically distinct roles for these isoforms have not been identified.
3. HIF-2α
Database searches for nucleotide sequences similar to those of HIF-1α cDNA resulted in the identification of a protein that was originally designated endothelial PAS domain protein 1 (EPAS1) (331), HIF-1α-like factor (HLF) (79), HIF-1α-related factor (HRF) (87), and member of PAS domain family 1 (MOP1) (117) but is now designated HIF-2α. HIF-1α and HIF-2α are the products of distinct genetic loci [HIF1A and EPAS1 (Unigene accession no. Hs.468410), respectively], and they share 48% amino acid identity overall with much higher identity in the bHLH (85%), PAS-A (68%), and PAS-B (73%) subdomains (331). HIF-2α can heterodimerize with HIF-1β, and the resulting transcription factor (HIF-2) can activate the expression of a reporter gene containing an HRE.
HIF-1α is expressed in all mammalian tissues and cell types analyzed (348, 351, 369). In contrast, HIF-2α expression is restricted to specific cell types, including developing blood vessels and lung (79, 87, 117, 331). Exposure of rats to hypoxia induces HIF-2α expression in distinct cell populations in brain, heart, intestine, kidney, liver, and pancreas (352). Whereas HIF-1α is present in both vertebrates such as Homo sapiens and invertebrates such as Caenorhabditis elegans (80, 145), HIF-2α appears to have arisen during vertebrate evolution.
Interestingly, Drosophila melanogaster contains a specialized system of O2 delivery consisting of tracheal tubes that conduct air from the exterior to the interior of the fly. The D. melanogaster genome encodes a HIF-1α homolog (designated Similar) and a HIF-1β homolog (designated Tango), which are ubiquitously expressed, and a second HIF-1α homolog (designated Trachealess), which is required for development of the tracheal tubes (129, 353). Thus insects and vertebrates appear to have independently evolved HIF-1α homologs (Trachealess and HIF-2α, respectively) that play important roles in directing specialized systems of O2 delivery.
Some HIF-1 target genes, such as EPO, GLUT1, VEGF, and EGLN3, can be activated by either HIF-1 or HIF-2, whereas other target genes, such as BNIP3, CAR9, LDHA, and PGK1, are only activated by HIF-1 (78, 123, 275, 318). Several target genes have been shown to be activated only by HIF-2, most notably genes encoding the stem cell markers NANOG, OCT4/POU5F1, and SOX2 in human embryonic stem cells (89). In human MCF-7 breast cancer cells, hypoxia induces binding of HIF-1 and HIF-2 to HREs in hundreds of target genes, but HIF-2 does not contribute to transactivation of these genes (78, 232). In mouse embryo fibroblasts (MEFs), HIF-2α is sequestered in the cytoplasm and does not contribute to hypoxia-induced gene transcription (251). Interaction of HIF-2α with the ETS family transcription factor ELK-1 may be required for HIF-2-dependent transactivation of some target genes in some cell types (2, 122). HIF-2α appears to play a particularly important role in the regulation of EPO gene expression and erythropoiesis as evidenced by loss-of-function mutation in mice (104) and gain-of-function mutation in humans (259).
4. HIF-3α
Database searches also revealed another HIF-1α paralog, originally called IPAS, which is now designated HIF-3α (208, 209). The HIF3A gene (Unigene accession no. Hs.420830) contains three alternative promoters, and multiple mature mRNAs are generated by utilization of different transcription initiation sites as well as alternative splicing of downstream exons (220). Some of the isoforms may dimerize with HIF-1β, whereas others appear to bind to HIF-1α. In both cases, the isoforms appear to inhibit HIF-1 transcriptional activity. HIF3A gene expression is induced by HIF-1 in hypoxic cells, suggesting that this may be a negative-feedback mechanism to attenuate HIF-1 activity during prolonged hypoxia (210, 211). HIF-3α may contribute to maintenance of the avascular state of the cornea by blocking the HIF-1-dependent expression of angiogenic growth factors (208).
C. Effect of Continuous Hypoxia on HIF Activity
1. Regulation of HIF-α protein stability by prolyl hydroxylation
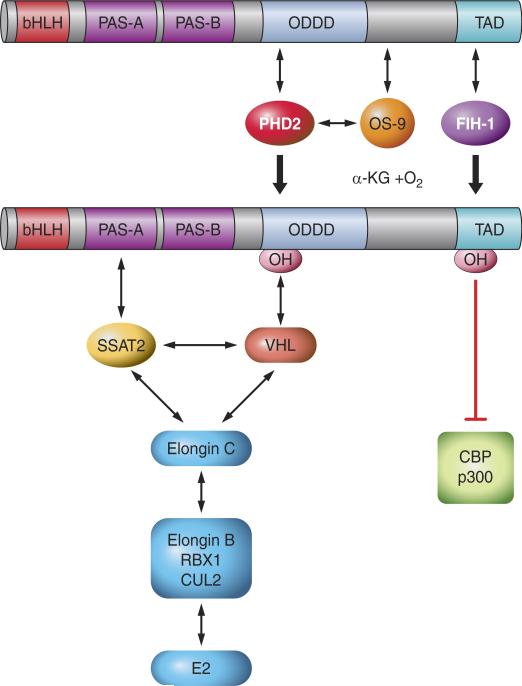
In well-oxygenated cells, HIF-α subunits (i.e., HIF-1α and HIF-2α) are bound by the von Hippel-Lindau protein (VHL), which recruits an E3-ubiquitin protein ligase consisting of Elongin 2, Elongin 3, Cullin 2, and RBX1 that is capable of functioning with E1-ubiquitin-activating and E2-ubiquitin-conjugating enzymes to mediate the ubiquitination of HIF-α (150, 219). Binding of VHL is conditional, based on the hydroxylation of proline-564 (in human HIF-1α) in well-oxygenated cells (134, 137, 371). Three prolyl-4-hydroxylase domain proteins (PHDs) that hydroxylate proline-564 in an O2-dependent manner were identified in mammalian cells (28, 80, 133). The proteins, which are designated PHD1, PHD2, and PHD3, are encoded by the EGLN2 (Hs.515417), EGLN1 (Hs.444450), and EGLN3 (Hs.135507) genes, respectively. The PHDs are members of a superfamily of dioxygenases that contain Fe(II) in their catalytic center and utilize O2 and α-ketoglutarate (2-oxoglutarate) as substrates. The reaction is as follows: PHD-Fe(II) + HIF-1α-Pro564 + O2 + α-ketoglutarate → PHD-Fe(III) + HIF-1α-HydroxyPro564 + CO2 + succinate. Reduction of Fe(III) to Fe(II) in the catalytic center by ascorbate is required for a subsequent catalytic cycle. The observed reduction in hydroxylase activity under hypoxic conditions may be explained by substrate (O2) limitation (49, 80, 108, 115, 225) and/or by an increase in mitochondrial production of reactive oxygen species (ROS) that may oxidize Fe(II) and inactivate the PHDs (29, 107, 212). HIF-1α is also hydroxylated at proline 402 (216), which requires prior hydroxylation of Pro564 (42). Hydroxylation induces binding of VHL at each of these proline residues (FIGURE 3). These two sites of prolyl hydroxylation share the consensus sequence LeuXxxXxxLeuAlaPro (80). Analogous proline residues are hydroxylated in HIF-2α (FIGURE 1) and are present within the same consensus sequence (216).
FIGURE 3.
Regulation of HIF-1α by O2-dependent hydroxylation. The prolyl hydroxylase PHD2 binds to HIF-1α and catalyzes the hydroxylation of Pro-402 and/or Pro-564. OS-9 stabilizes PHD2-HIF-1α interaction and promotes hydroxylation. VHL binds to prolyl hydroxylated HIF-1α and recruits the ElonginC-ElonginB-CUL2-RBX1 E3-ubiquitin ligase. SSAT2 stabilizes HIF-1α-VHL-ElonginC complex formation, thereby promoting ubiquitination. FIH-1 binds to HIF-1α and catalyzes the hydroxylation of Asn-803, which blocks the binding of the coactivators p300 and CBP to the TAD-C.
2. Regulation of HIF-α TAD function by asparaginyl hydroxylation
FIH-1 (factor inhibiting HIF-1) was identified in a yeast two-hybrid screen and shown to bind to amino acid residues 757– 826 of HIF-1α, leading to repression of HIF-1-dependent transcription (207). Hydroxylation of Asn-803 located in the TAD-C (FIGURE 3) blocks the binding of the coactivators p300 and CBP (181). FIH-1 is the asparaginyl hydroxylase (180). As in the case of the prolyl hydroxylases, FIH-1 utilizes O2 and 2-oxogluta-rate and contains Fe(II) in its catalytic center (60, 185, 224). FIH-1 has a Km for O2 (using HIF-1α peptides as substrates for in vitro hydroxylation) that is three times lower than the prolyl hydroxylases (161). FIH-1 also interacts with VHL (207), which may stabilize its binding to HIF-1α under nonhypoxic conditions. Whereas the expression of most HIF-1 target genes is negatively regulated by FIH-1, there is a subset of genes (including BNIP3 and PGK1) whose expression is independent of FIH-1, suggesting that transactivation of these genes is dependent only on TAD-N (61). Although FIH-1 hydroxylates both HIF-1α and HIF-2α, its activity for HIF-1α is significantly greater (24). As a result, HIF-1 transcriptional activity is intrinsically more O2-labile.
3. Regulation of HIF-1α mRNA levels
HIF-1α mRNA levels increase dramatically in response to hypoxia or ischemia in brain, heart, kidney, lungs, and skeletal muscle (15, 23, 189, 351, 369). It is not known whether the primary mechanism is increased mRNA transcription, decreased mRNA degradation, or both. In ischemic skeletal muscle, the induction of HIF-1α mRNA appears to be HIF-1α dependent (23). One possibility is that HIF-1α mRNA stability is regulated by microRNAs (miRs), which bind to the 3′-untranslated region of mRNAs and either block their translation or induce their degradation. In cardiac myocytes subjected to hypoxia, levels of miR-199a decreased, which was required for the induction of HIF-1α protein levels (274); however, HIF-1α mRNA levels were not analyzed in this study so it is not clear whether miR-199a regulated HIF-1α mRNA translation or stability. In contrast to what is observed in vivo, HIF-1α mRNA levels are not induced by hypoxia in most tissue culture cell lines, which represents a major obstacle to understanding the regulatory mechanism that is active in vivo.
D. O2-Independent Regulation of HIF Activity
1. Signal transduction pathways involving protein phosphorylation
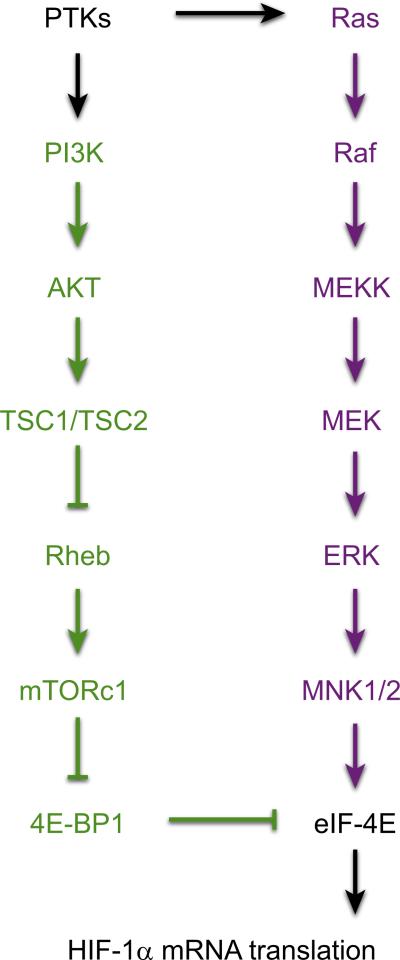
The first evidence that stimuli other than hypoxia could induce HIF-1 was the finding that overexpression of the v-Src oncoprotein increased HIF-1α protein, HIF-1 transcriptional activity, and expression of the HIF-1 target gene Eno1 in mouse 3T3 cells (141). Human pulmonary artery smooth muscle cells (PASMCs) were the first nontrans-formed cell type found to express high levels of HIF-1α protein and HIF-1 DNA-binding activity under nonhypoxic conditions (369). Acute exposure of cultured cells to growth factors, including epidermal growth factor (EGF), fibroblast growth factor 2, insulin, insulin-like growth factor (IGF)-I, and IGF-II, was shown to increase HIF-1α protein levels (83, 376). Dozens of cytokines and growth factors are now known to increase HIF-1α protein levels in a cell type-specific manner, which is dependent on expression of the cognate receptor and appropriate downstream signal transduction pathway components (FIGURE 4).
FIGURE 4.
Signal transduction pathways leading to increased HIF-1α protein levels. Activation of protein tyrosine kinases (PTKs) by mutation or ligand binding leads to signaling via the phosphatidylinositol 3-kinase (PI3K) pathway (green) and MAP kinase pathway (purple) that ultimately stimulates the activity of eukaryotic translation initiation factor 4E (eIF-4E) or blocks the activity of its inhibitor, eIF-4E binding protein 1 (4E-BP1), leading to increased translation of HIF-1α mRNA into protein.
If physiological stimuli other than hypoxia can increase HIF-1α levels and HIF-1 activity, then it follows that high levels of HIF-1α may not be sufficient evidence to conclude that a given cell or tissue is hypoxic. More unequivocal evidence can be obtained by direct measurement of tissue Po2 levels (31, 128, 250, 354, 382) or by pimonidazole staining of tissue sections (339).
In human prostate cancer cells, constitutive expression of HIF-1α was found to require signal transduction via phosphatidylinositol-3-kinase (PI3K), AKT (protein kinase B), and the mammalian target of rapamycin (mTOR; also known as FKBP/rapamycin binding protein), a serine/threonine protein kinase that phosphorylates p70 ribosomal protein S6 kinase 1 (S6K1) and the eIF-4E binding protein 1 (4E-BP1), thereby stimulating protein synthesis (383). Analysis of IGF-I-treated colon cancer cells and insulin-treated retinal epithelial cells revealed that activation of PI3K was required to induce increased HIF-1α protein levels (92, 333). When MCF-7 human breast cancer cells were treated with the EGF family member heregulin, PI3K/AKT signaling led to activation of mTOR, which stimulated the rapamycin-sensitive translation of HIF-1α mRNA into protein, and this effect was dependent on the presence of 5′-untranslated sequences in HIF-1α mRNA (184).
Recent studies have revealed that association of mTOR with Raptor or Rictor defines two different mTOR-containing signaling complexes, which are designated mTORC1 and mTORC2, respectively (182). In renal carcinoma cells, HIF-1α expression is dependent on both mTORC1 (which phosphorylates S6K1 and 4E-BP1) and mTORC2 (which phosphorylates AKT), whereas HIF-2α expression is dependent only on mTORC2 (332). In some cell types, mTOR may also promote HIF-1α stabilization and/or TAD function, although the molecular mechanisms are not completely understood (127).
In addition to acting through the PI3K/AKT/mTOR pathway, IGF-I-mediated HIF-1α expression also utilized the MAP kinase signal transduction pathway (92). The MAP kinases ERK1 and ERK2 phosphorylate and activate the MAP kinase-interacting kinases MNK1 and MNK2, which directly phosphorylate eIF-4E (FIGURE 4), thereby promoting the formation of the eIF-4F complex that initiates translation (271). Taken together, these results established the existence of extensive and important crosstalk between oxygen sensing and signal transduction pathways in mammalian cells.
2. Nitric oxide, prostaglandins, and metabolites
Small molecule messengers, such as nitric oxide (NO) and prostaglandin E2 (93, 158), as well as metabolites, such as lactate, fumarate, and succinate (131, 202, 296), also increase the levels of HIF-1α protein under nonhypoxic conditions. Prostaglandin E2 binds to G protein-coupled cell surface receptors that activate the PI3K and MAP kinase pathways (93) as described above. Several different mechanisms of action have been proposed for induction of HIF-1α by NO, including S-nitrosylation of HIF-1α protein (192, 248), activation of the PI3K pathway (152, 286), inhibition of prolyl hydroxylase activity (227), and redistribution of intracellular O2 due to inhibition of mitochondrial respiration (108). Metabolite induction of HIF-1α results from inhibition of PHD2 activity (114, 131, 162, 201, 296). The tricarboxylic acid (TCA) cycle intermediates fumarate and succinate are particularly interesting because they have been reported to inhibit PHD2 but not FIH-1 (114).
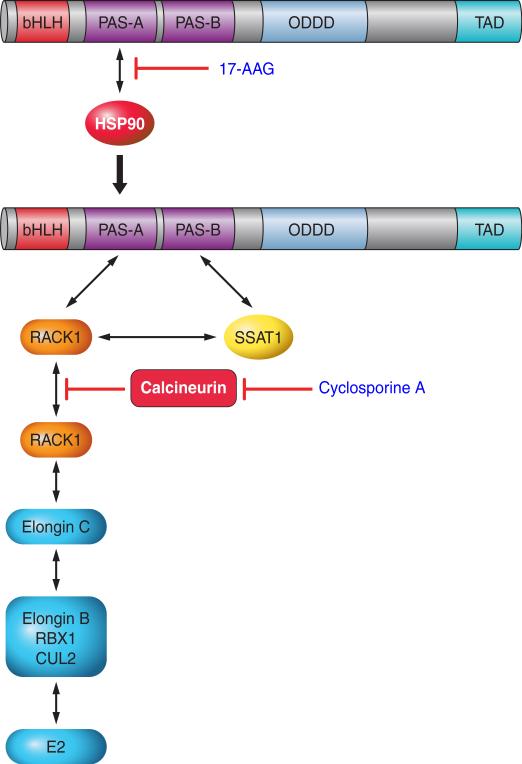
3. HSP90, RACK1, and calcineurin
Heat shock protein 90 (HSP90) is a molecular chaperone that protects client proteins from misfolding and degradation (349). HIF-1α interacts with HSP90, which is required for the induction of HIF-1α in response to hypoxia (230). The stability of HIF-1α appears to be dependent on its interaction either with HSP90 or HIF-1β (132). Inhibitors of HSP90, such as geldanamycin and its derivative 17-allylaminogeldanamycin (17-AAG), have anti-cancer effects and have been shown to induce the ubiquitination and proteasomal degradation of HIF-1α (FIGURE 5) even in cells that lack VHL (130). Binding of 17-AAG to HSP90 displaces it from the PAS-B subdomain of HIF-1α, thereby allowing the protein RACK1 to bind to HIF-1α, and recruit an Elongin C ubiquitin-ligase complex, which ubiquitinates HIF-1α (197). This pathway is also modulated by calcineurin, a Ca2+- and calmodulin-dependent serine-threonine phosphatase (FIGURE 5). Elongin C and HIF-1α each bind to the same site on RACK1, and dimerization of RACK1 is required to recruit Elongin C to HIF-1α; phosphorylation of RACK1 promotes its dimerization and dephosphorylation by calcineurin inhibits dimerization (198). This pathway may be of clinical relevance because treatment of rats with the calcineurin inhibitor cyclosporine A blocks HIF-1 target gene expression and the development of hypoxic pulmonary hypertension (166), which is a HIF-dependent disorder (30, 370), as described below in section VII.
FIGURE 5.
Ubiquitination of HIF-1α by RACK1. Inhibitors of HSP90, such as 17-allylamino-17-demethoxygeldanamycin (17-AAG), block the binding of HSP90 to the PAS-A domain of HIF-1α, allowing RACK1 to bind at this site and recruit the ElonginC ubiquitin ligase complex. Calcineurin dephosphorylates RACK1, thereby preventing its dimerization, which is necessary to bring the ElonginC complex into contact with HIF-1α. Calcineurin activity is inhibited by cyclosporine A, which thereby increases ubiquitination of HIF-1α in a RACK1-dependent manner.
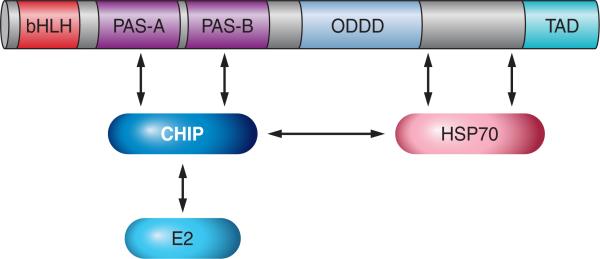
4. HSP70 and CHIP
HIF-α subunits are stabilized under conditions of acute hypoxia. However, prolonged hypoxia leads to decay of HIF-1α, but not HIF-2α, protein levels by unknown mechanisms (118, 336). HIF-1α was found to bind to both HSP70 and the COOH terminus of Hsc70-interacting protein (CHIP), which is an Hsp70-associated E3 ubiquitin ligase, resulting in the ubiquitination and proteasomal degradation of HIF-1α (FIGURE 6). Although HSP70 binds to HIF-2α, CHIP does not, and as a result, HIF-2α is resistant to HSP70/CHIP-mediated degradation (206). These results thus provide a molecular mechanism underlying the preferential stabilization of HIF-2α under conditions of prolonged hypoxia.
FIGURE 6.
Ubiquitination of HIF-1α by CHIP. HSP70 recruits CHIP and its associated E2 ligase to HIF-1α.
5. Hypoxia-associated factor
Hypoxia-associated factor (HAF) binds to HIF-1α and promotes its ubiquitination and proteasomal degradation, whereas HAF binding to a different site on HIF-2α promotes its transcriptional activity without affecting its stability, providing another mechanism for the switch from HIF-1 to HIF-2 activity under conditions of chronic hypoxia (160).
E. Molecular Mechanisms by Which HIFs Mediate Biological Effects
There are multiple mechanisms by which HIF-1α and HIF-2α mediate adaptive physiological responses to hypoxia. These fall into two groups: those that involve the formation of HIF-α:HIF-1β heterodimers and those that are mediated by HIF-1α acting as a monomer. Biological effects that are mediated by the heterodimerization of HIF-1β (ARNT) with other bHLH-PAS proteins such as the AHR (365) are not discussed here.
1. Binding of HIF heterodimers to HREs in target genes
As in the case of the EPO gene described in section II, HIF-1 and HIF-2 bind to the sequence 5′-(A/G)CGTG-3′ located within HREs of target genes to activate their transcription. With the use of microarray gene chips, it is possible to identify hundreds of mRNAs whose expression is increased when human cell lines are exposed to hypoxia for 24 h. If one adds to this experiment the use of RNA interference technology, one can show that the vast majority of these changes in gene expression are HIF dependent. If one then adds a third new technology, whole-genome chromatin immunoprecipitation (ChIP) assays, it is possible to demonstrate the hypoxia-induced binding of HIF-1 to the same genes whose mRNA levels are increased in response to hypoxia. This so-called ChIP-chip experiment allows one to reliably identify the direct target genes of HIF-1 in any given cell type (232, 357).
Remarkably, microarray assays have revealed that the levels of hundreds of other mRNAs are decreased in response to hypoxia, and although these changes in gene expression are also HIF-1 dependent, ChIP assays indicate that HIF-1 does not bind directly to the genes that are hypoxia-repressed (232). There are at least three possible mechanisms by which HIF-1 may repress gene expression in hypoxic cells: 1) HIF-1 may bind to and transactivate a target gene encoding a transcriptional repressor. The repressor, once translated, will bind to its own target genes and repress their transcription. Since this transcriptional repression is dependent on HIF-1, the direct targets of the repressor may be considered indirect or secondary targets of HIF-1. Because transcription factors represent the largest single category of proteins encoded by HIF-1 target genes (211), the number of indirect targets may be considerable. 2) HIF-1 may bind to and transactivate a target gene encoding one or more miRs, which once transcribed and processed, would bind to their target mRNAs and promote their degradation. The expression of a large and growing number of miRs is induced by hypoxia through HIF-1 (58). 3) A few genes appear to be directly repressed by the binding of HIF-1 to a so-called “reverse HRE” containing the consensus sequence 5′-YGCAC-3′ (139, 187, 221, 240).
2. Binding of HIF-1α to other transcription factors
HIF-1α can also regulate gene expression without binding to DNA at all. There are now several examples in which HIF-1α binds to other transcription factors and blocks the assembly of transactivation complexes (164, 165). In other cases, HIF-1α binds to transcription factors and enhances their transactivation function (106). These effects of HIF-1α do not require the presence of HIF-1β.
F. Positive and Negative Feedback Loops
In hypoxic cells, HIF-1 activates the transcription of hundreds of target genes. These target genes encode proteins (e.g., EPO and VEGF) that mediate adaptive responses to hypoxia (erythropoiesis and angiogenesis, respectively). However, these gene products may also modulate HIF-1 activity. The large number of known feedback loops, some of which are listed in TABLE 2, underscores the critical importance of precisely regulating O2 delivery and utilization. Many of these feedback loops are only active in certain cell types and thus provide a means to customize physiological responses to hypoxia.
Table 2.
HIF-1 positive and negative feedback pathways
| HIF-1 Target | Feedback | Cell Type(s) | Reference Nos. |
|---|---|---|---|
| ACE, AT1R | Positive | PA fibroblast | 169 |
| AS-HIF-1α | Negative | Renal carcinoma | 329 |
| Various tissues | 282 | ||
| CITED2 | Negative | U2-OS, Hep3B | 17 |
| COX2 | Positive | HCT116 | 93 |
| HT29 | 148 | ||
| CSB | Positive | Fibroblast | 85 |
| FOXO3A | Negative | MEF, 3T3 | 7 |
| HIF-3α/IPAS | Negative | Brain EC | 210 |
| IGF-II | Positive | 293, MEF | 83 |
| 320 | |||
| JMJD1A | Positive | RCC4 | 170 |
| miR-20b | Mut Antag | H22 | 190 |
| NEDD9 | Positive | LS174T | 157 |
| NOS2 | Positive | ANA-1 | 226 |
| Oral SCC | 272 | ||
| NOX2 | Positive | Brain | 257 |
| PDGF-B | Positive | Hepatocellular cancer | 183 |
| PDK1 | Positive | Head/neck SCC | 223 |
| PHD2 | Negative | C6 | 59 |
| Glioma | 113 | ||
| PKM2+PHD3 | Positive | HeLa, Hep3B | 205 |
| RAC1 | Positive | PA smooth muscle cell | 68 |
| RBX2 | Negative | MEF, DLD-1 | 325 |
| REDD1 | Negative | MEF | 120 |
| SENP1 | Positive | EC | 360 |
PA, pulmonary artery; MEF, mouse embryo fibroblast; EC, endothelial cell; Mut Antag, mutual antagonism; SCC, squamous cell carcinoma.
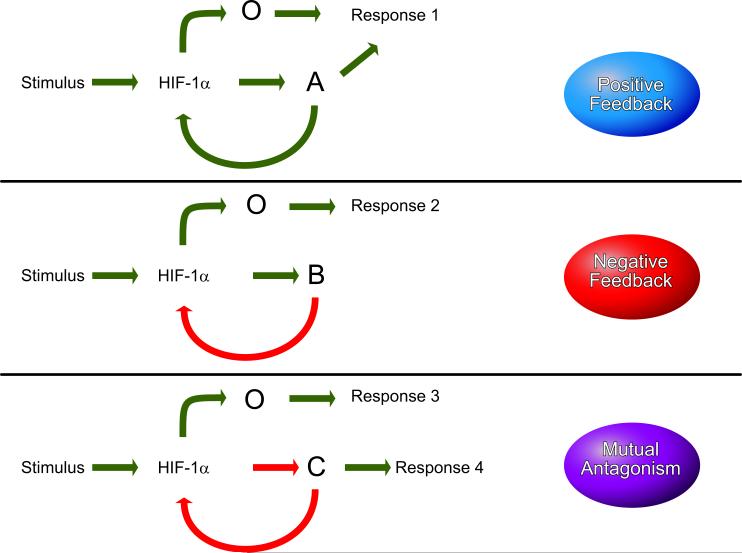
Positive feedback (also known as a feed-forward mechanism) serves to amplify the response to hypoxia and is likely tobeemployedwhenrapidresponsesarerequired(FIGURE 7, top panel). Negative feedback, on the other hand, may provide a mechanism to prevent overshoot phenomena (FIGURE 7, middle panel). For example, after hemorrhage, EPO mRNA and protein levels increase to stimulate red blood cell production, but then decline, prior to any recovery of the red blood cell count, indicating a negative feedback loop in the EPO-producing cells that is independent of Po2. This negative feedback prevents excess erythrocytosis, which might result in stroke due to sludging of red blood cells in cerebral blood vessels. The gene product need not be a protein: a HIF-1α antisense mRNA is produced under hypoxic conditions and may downregulate the levels of HIF-1α protein (329). The microRNA miR-20b inhibits HIF-1α expression, whereas HIF-1 represses miR-20b expression, indicating a mutually antagonistic relationship (FIGURE 7, bottom panel).
FIGURE 7.
Mechanisms for feedback regulation of HIF-1 by target gene products. Stimulatory and inhibitory signals are shown in green and red, respectively. Top panel: in a positive feedback loop, HIF-1 activates the transcription of gene A and other (O) genes. The protein product of gene A stimulates HIF-1 transcriptional activity. Middle panel: in a negative-feedback loop, HIF-1 activates the transcription of gene B. The protein product of gene B inhibits HIF-1 transcriptional activity. Bottom panel: in a mutually antagonistic feedback loop, HIF-1 inhibits the expression of gene C. The protein product of gene C inhibits the activity of HIF-1.
An interesting feed-forward mechanism involves the selective stimulation of HIF-2α (but not HIF-1α) transactivation domain function by the redox-regulated deacetylase SIRT1, which binds to HIF-2α and may catalyze deacetylation of one or more lysine residues (69). SIRT1 transcription in hypoxic cells is activated by HIF-1 and HIF-2, with SIRT1 protein stimulating HIF-2-dependent SIRT1 gene transcription (47). In cardiac myocytes, SIRT1 inhibits transcription of the EGLN1 gene (which encodes the prolyl hydroxylase PHD2), thereby reducing the degradation of HIF-1α and HIF-2α (274).
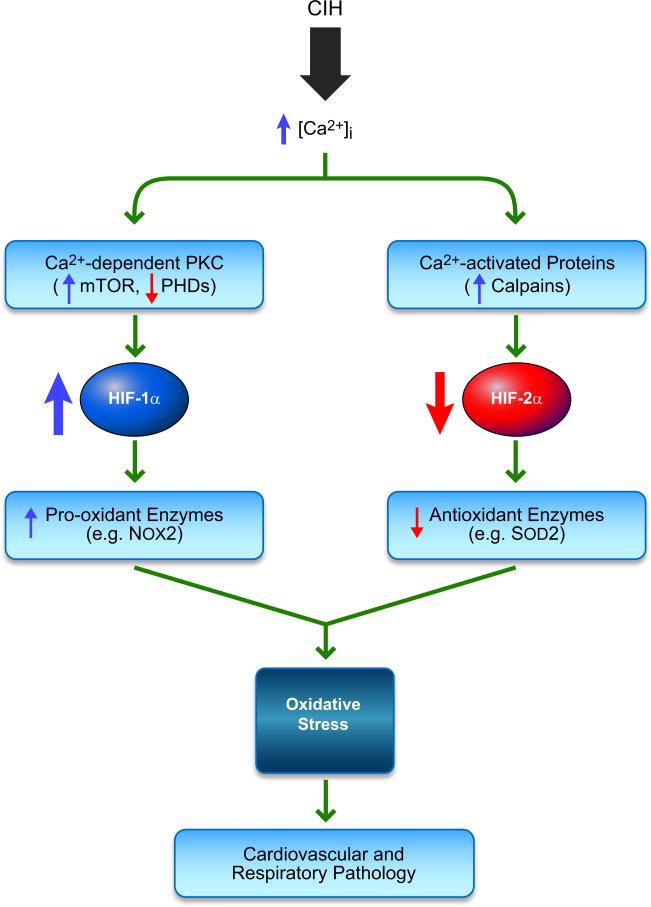
The molecular mechanism of feedback by proteins that are encoded by HIF-1 target genes can also vary. For example, the protein can bind directly to HIF-1α, as in the case of PHD2, or to a HIF-1α regulator, as in the case of CITED2, which competes with HIF-1α for binding to the coactivator CBP (TABLE 2). Alternatively, the protein may have a catalytic activity, and the reaction product may regulate HIF-1, as in the case of COX2 and NOS2, which produce prostaglandin E2 and NO, respectively. The critical role of the feed-forward relationship between HIF-1 and NOX2, an NADPH oxidase that generates superoxide anions, in the cardiorespiratory pathology induced by chronic intermittent hypoxia will be discussed in detail in section X.
What is the significance of the many mechanisms (O2-dependent and O2-independent, feedback and feed-forward) for regulating HIF activity that have been described in section II? Multiple mechanisms provide a means for integration of various critical physiological stimuli, including O2, ROS, metabolites, cytokines, and growth factors, at the level of HIF activity. Second, multiple mechanisms provide flexibility: within any given cell, a particular stimulus-response pathway may be given priority over others. The take-home message: HIF signaling is not a linear pathway, but a complex web with perhaps hundreds of input stimuli and thousands of potential output responses, with each representing a different target gene.
III. CELL AUTONOMOUS METABOLIC RESPONSES TO HYPOXIA
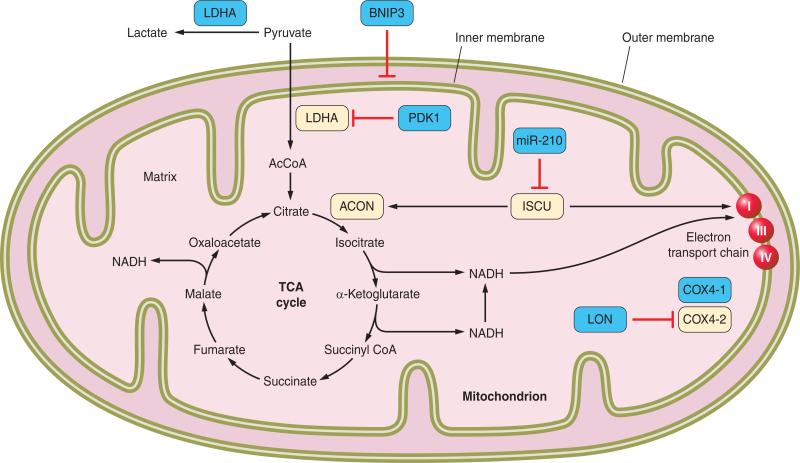
HIF-1 is present in all metazoan species, even the simple roundworm C. elegans, in which O2 diffuses from the environment to all cells and there are no specialized systems for O2 delivery. The primordial function of HIF-1 appears to involve finding the optimal balance between oxidative and glycolytic metabolism for any given cell as a function of the local O2 concentration. A key decision point for the metabolism of glucose involves the fate of pyruvate, which can be converted by pyruvate dehydrogenase (PDH) into acetyl coenzyme A (AcCoA) and thereby serve as a substrate for oxidation via the TCA cycle; alternatively, lactate dehydrogenase A (LDHA) can convert pyruvate into lactate, the glycolytic end product that is exported from the cell (FIGURE 8). Under hypoxic conditions, HIF-1 activates the transcription of the genes encoding LDHA (298) and PDH kinase 1 (PDK1) (156, 249), which phosphorylates the catalytic subunit of PDH and inactivates it. As a result, there is a shift from oxidative to glycolytic metabolism of pyruvate. To compensate for the greatly reduced efficiency of ATP generation associated with glycolytic rather than oxidative metabolism, HIF-1 also activates transcription of the genes encoding glucose transporters and glycolytic enzymes, thereby increasing flux through the pathway (135, 294). In hypoxic cancer cells, transcription of the PKM2 gene encoding pyruvate kinase M2 is activated by HIF-1. In addition to its activity as a glycolytic enzyme, PKM2 binds to HIF-1α and stimulates its transactivation function, a feed-forward mechanism that promotes the switch from oxidative to glycolytic metabolism (205).
FIGURE 8.
Regulation of glucose metabolism by HIF-1. The blue boxes indicate products of HIF-1 target genes, which are induced under hypoxic conditions. α-KG, α-ketoglutarate; AcCoA, acetyl coenzyme A; ETC, electron transport chain; TCA, tricarboxylic acid.
It is commonly assumed that the switch from oxidative to glycolytic metabolism in cells exposed to 1% O2 (7 mmHg) is because O2 is a limiting substrate for cellular respiration (and thus for ATP synthesis) under these conditions. However, HIF-1α null [knockout (KO)] MEFs, which do not switch from oxidative to glycolytic metabolism under hypoxic conditions, have higher ATP levels at 1% O2 than WT cells have at 20% O2 (140 mmHg), indicating that 1% O2 is not limiting for oxidative phosphorylation (378). Instead, the activation of PDK1 expression by HIF-1 is required to maintain redox homeostasis. Under conditions of chronic hypoxia, WT MEFs reduce their levels of ROS, while in HIF-1α-KO cells, ROS increase to levels that result in cell death (156). Thus ATP generation can be maintained in MEFs under hypoxic conditions but at the cost of generating toxic levels of ROS.
Oxidation of fatty acids in the mitochondria also generates AcCoA to fuel oxidative metabolism, and this pathway is not affected by PDK1 or LDHA. Thus another strategy to prevent the generation of excess mitochondrial ROS in hypoxic cells is to reduce mitochondrial mass through autophagy. HIF-1 triggers selective mitochondrial autophagy through transcriptional activation of the gene encoding BNIP3, a mitochondrial protein that competes with Beclin 1 for binding to Bcl2, thereby freeing Beclin 1 to associate with phosphatidylinositol 3-kinase and trigger selective mitochondrial autophagy (378). HIF-1α-KO MEFs can be rescued from hypoxic cell death by forced expression of either PDK1 (156) or BNIP3 (378). HIF-1-regulated BNIP3L expression also contributes to autophagy in many cell types (14).
Perhaps the most compelling evidence that HIF-1 plays a critical role in modulating ROS as a function of O2 concentration is the discovery that HIF-1 orchestrates a subunit switch in the composition of cytochrome c oxidase (COX; complex IV of the electron transport chain) under hypoxic conditions (94). The switch from utilization of the COX4–1 to the COX4–2 subunit appears to fine-tune complex IV activity to optimize its efficiency under hypoxic conditions. Compared with the adaptive metabolic responses mediated by PDK1 and BNIP3, this is a more subtle response, suggesting that it may be activated first, thereby allowing oxidative phosphorylation to continue under conditions of mild hypoxia. The unicellular eukaryote Saccharomyces cerevisiae, which lacks HIF-1, also responds to hypoxia by switching COX subunits, but by a completely different mechanism (175), suggesting that regulation of COX activity as a function of O2 concentration is a requirement for all eukaryotic cells.
The complex regulation of oxidative and glycolytic metabolism by HIF-1 further underscores the importance of these adaptive responses for cell survival (FIGURE 8). In addition to the direct regulation of genes encoding PDK1, BNIP3, COX4–2, and LON (a mitochondrial protease that is required for degradation of COX4–1), HIF-1 also activates transcription of the gene encoding microRNA miR-210, which inhibits expression of the iron-sulfur cluster assembly proteins ISCU1/2, which are required for activity of the TCA cycle enzyme aconitase and electron transport chain (ETC) complex I (43).
Acute exposure of cells to hypoxia results in an increased rate of ROS generation by mitochondrial ETC complex III, which appears to contribute to the inhibition of PHD2 activity, thereby activating HIF-1 (29, 107, 212), which in turn mediates adaptive responses that result in reduced mitochondrial ROS generation (156, 378). As such, this represents a classical stimulus-response paradigm that links oxygen, energy, and redox homeostasis, the maintenance of which is essential for cell survival. However, it should be noted that other investigators have reported that inhibitors of any of the ETC complexes will inhibit the hypoxia-induced stabilization of HIF-1α and that they do so by reducing mitochondrial O2 consumption, thereby facilitating prolyl hydroxylation (49, 73, 108).
IV. PRECONDITIONING PHENOMENA
When tissue perfusion is interrupted, the ensuing ischemia will ultimately result in tissue death. For most organs, including heart, kidney, and liver, 30 min of ischemia followed by reperfusion is sufficient to cause cell death, whereas the critical ischemic period in the brain is only 5 min. Exposure of the heart to several repeated cycles of brief (5-min) episodes of ischemia followed by equally brief periods of reperfusion protects against myocardial infarction following a subsequent prolonged (30-min) episode of ischemia, a phenomenon known as ischemic preconditioning (237). Preconditioning results in an acute (early) phase of protection immediately after the preconditioning stimulus that lasts for several hours and a delayed (late) phase starting 24 h after the preconditioning stimulus that lasts for several days (213). Perfusion of the heart with hypoxic blood (308) or adenosine analogs (196) also induces protection. Exposure of rats to ambient hypoxia [3 wk at 10% O2 (70 mmHg)] also protects the heart against ischemiareperfusion injury (324).
Exposure of mice to alternating cycles of ambient hypoxia [6% O2 (42 mmHg) for 6 min] and reoxygenation [21% O2 (147 mmHg) for 6 min] for 1 h induced late-phase cardiac preconditioning, and this protective effect of hypoxia-reoxygenation was lost in Hif-1α+/− mice, which are heterozygous for the HIF-1α-KO allele (34). In these mice, exposure to cycles of hypoxia and reoxygenation induced EPO expression in the kidney and its secretion into the bloodstream. EPO perfusion in Langendorff heart preparations resulted in acute protection against ischemia-reperfusion injury (35). The HIF-1-dependent production in hypoxic/ischemic tissues of secreted survival factors such as EPO is likely to contribute to remote ischemic preconditioning, which refers to the observation that ischemia in one organ/tissue is protective against subsequent ischemia in a distant organ/tissue (155).
When Langendorff-perfused hearts from WT mice were exposed to two cycles of ischemia for 5 min and reperfusion for 5 min (i.e., I5R5I5R5), they were acutely protected against myocardial injury when subsequently subjected to prolonged ischemia (30 min) and reperfusion. In contrast, exposure of hearts from Hif-1α+/− mice to the I5R5I5R5 has no protective effect (36). However, infusion of adenosine induced protection of hearts from both WT and Hif-1α+/− mice, indicating a specific defect in ischemic preconditioning (36). Administration of small interfering RNAs that inhibited HIF-1α expression in the heart also eliminated ischemic preconditioning (76).
Adenosine levels were found to be increased in the heart following ischemic preconditioning, and adenosine receptor antagonists blocked the protective effect of ischemic preconditioning, while administration of adenosine receptor agonists was sufficient to induce protection against myocardial injury following ischemia-reperfusion (328). Ectonucleoside triphosphate diphosphohydrolase (ENTPD1 or CD39) metabolizes ATP to ADP and then to AMP and ecto-5′-nucleotidase (NT5E or CD73) metabolizes AMP to adenosine. CD73 expression is induced under hypoxic conditions as a result of HIF-1-dependent transcriptional activation of the Nt5e gene (321). Mice with CD73 deficiency lack ischemic preconditioning (77), similar to Hif-1α+/− mice, and Hif-1α+/− hearts were protected against myocardial ischemia-reperfusion injury by adenosine perfusion (36). Thus insufficient adenosine production, resulting from deficiency of HIF-1 (or CD73), leads to the loss of preconditioning, which can be rescued pharmacologically by adenosine treatment. Although this model is consistent with the experimental data described above, it is likely that HIF-1 mediates additional protective responses to an ischemic preconditioning stimulus, including adaptive changes in cell metabolism such as those described above in section III.
V. VENTILATORY ADAPTATION TO HYPOXIA: O2 SENSING BY THE CAROTID BODY
A. Responses of the Carotid Body to Acute Hypoxia
Carotid bodies are specialized sensory organs for monitoring changes in arterial blood O2. Systemic hypoxia stimulates breathing and raises blood pressure within seconds after its onset. These responses are mediated by neural reflexes, which arise in the carotid body. Hypoxia augments the sensory discharge of the carotid body in a stimulus-dependent manner in all mammalian species studied (172). The carotid body is composed of two major cell types: type I and type II. Type I cells (also called glomus cells) are of neural crest origin and express a variety of neurotransmitters, whereas type II cells (also called sustentacular cells) resemble glial cells of the central nervous system (172). Sensory innervation of the carotid body comes from the glossopharyngeal nerve, whose cell bodies reside in the petrosal ganglion. Available evidence indicates that the type I cells are responsible for O2 sensing and that they work in concert with the opposing afferent nerve ending as a sensory unit.
1. HIF-1α and HIF-2α expression in the carotid body
Type I cells of the rat and mouse carotid bodies express HIF-1α, as evidenced by colocalization with tyrosine hydroxylase, a marker of type I cells (159, 179, 283), as do some type II cells (283). HIF-1α expression is more abundant in fetal and neonatal carotid bodies, and its expression decreases during the postnatal period (283), which may reflect the increased arterial Po2 after birth. On the other hand, HIF-2α is expressed predominantly in type I cells (253, 283, 330), and its expression exhibits little developmental variation (283).
2. Role of HIFs in O2 sensing by the carotid body
Carotid bodies from Hif-1α+/− mice exhibit remarkable impairment of the hypoxic response, and this defect is selective because the response to cyanide, a potent chemoreceptor stimulant, was unaffected in these mice (159). Carotid body morphology of Hif-1α+/− mice was comparable with WT mice, indicating that the impaired hypoxic sensitivity was not secondary to changes in carotid body structure. These findings demonstrate that even partial deficiency of HIF-1α profoundly impacts hypoxic sensing by the carotid body. The mechanism by which HIF-1 contributes to acute hypoxic sensing by the carotid body will be discussed below.
Chuvash polycythemia (CP) is an autosomal recessive human disorder in which the O2-dependent degradation of HIF-α subunits is impaired due to a missense mutation in VHL, resulting in elevated HIF activity at any given O2 concentration. CP subjects exhibit augmented ventilatory and heart rate responses to acute hypoxia (313). Given that chemosensory reflexes mediate autonomic responses to hypoxia, the heightened cardiorespiratory changes seen in CP patients may be due to elevated HIF activity in the carotid body. Thus studies on mice with loss of function and CP subjects with gain of function suggest that HIF-1α plays a critical role in carotid body responses to acute hypoxia.
A recent study examined the hypoxic response of the carotid body in Hif-2α+/− mice (253). Like Hif-1α+/− mice, carotid body morphology in Hif-2α+/− mice was comparable to WT littermates. In striking contrast to Hif-1α+/− mice, Hif-2α+/− mice manifested heightened carotid body responses to hypoxia, whereas responses to hypercapnia or cyanide, which are potent carotid body stimulants, were comparable to WT littermates. These observations suggest that partial deficiency of HIF-2α leads to selective potentiation of the hypoxic response of the carotid body. Antioxidants prevented the exaggerated hypoxic sensitivity in Hif-2α+/− mice. Carotid bodies from Hif-2α+/− mice showed reduced levels of mRNAs encoding antioxidant enzymes and oxidative stress (253), suggesting that heightened hypoxic sensitivity of the carotid body is due to oxidative stress.
Although HIF-2α shares several common features with HIF-1α including 48% amino acid sequence identity (79), Hif-1α+/− and Hif-2α+/− mice show striking differences in carotid body responses to hypoxia. HIF-1α deficiency results in hyposensitivity, whereas HIF-2α deficiency results in hypersensitivity of chemoreceptor responses to acute hypoxia. On the basis of these observations, it was proposed that HIF-2α plays an antagonistic rather than redundant role to that of HIF-1α in regulating O2 sensing by the carotid body (253).
The analysis of mice with targeted deletion of the gene encoding the HIF-1α asparaginyl hydroxylase FIH-1 (see sect. IIC2) revealed no discernable defects in erythropoiesis or angiogenesis, but the mice exhibited hypermetabolism and hyperventilation, which were attributed to enhanced carotid body sensitivity to hypoxia (380). Only a subset of HIF target genes are sensitive to regulation by FIH-1 (61), and it will be interesting to determine whether changes in the expression of specific target genes in the carotid body can be identified in mice deficient for FIH-1.
B. Carotid Body Response to Chronic Hypoxia
Continuous hypoxia such as that experienced during sojourns at high altitude leads to a series of physiological adaptations. Ventilatory acclimatization to hypoxia (VAH) represents one such adaptation (263). VAH is characterized by a progressive increase in baseline ventilation and augmented ventilatory response to subsequent acute hypoxia (288, 341). VAH is an important physiological adaptation because failure to hyperventilate at high altitude leads to severe hypoxemia. A large body of evidence suggests that carotid body stimulation and the ensuing chemosensory reflex are critical for evoking VAH (9, 19, 63, 266, 280, 340).
1. HIF-1 and ventilatory adaptation to continuous hypoxia
Ventilatory adaptation to chronic hypoxia was investigated in Hif-1α+/− mice (159). Following hypobaric hypoxia, WT mice exhibited augmented hypoxic ventilatory responses, which is a hallmark of ventilatory acclimatization. In striking contrast, Hif-1α+/− mice displayed remarkable absence of ventilatory acclimatization and in fact showed reduced hypoxic ventilatory stimulation following chronic hypoxia. Similar loss of ventilatory acclimatization was also found in mice with targeted disruption of HIF-1α function in the brain stem neurons associated with reflex stimulation of breathing (262). CP patients, who have increased HIF-1α levels, displayed increased baseline ventilation and markedly enhanced ventilatory responses to acute hypoxia (313). Together these studies provide evidence that HIF-1 is a critical mediator of ventilatory adaptation to chronic hypoxia.
2. Chronic hypoxia and HIF-1α expression in the chemosensory reflex pathway
Chronic hypoxia increases HIF-1α levels in the nuclei and cytoplasm of glomus cells as well as endothelial cells of the carotid body (179, 283). As short as 1 h of hypoxia is adequate to cause HIF-1α accumulation in glomus cells (6), and the elevated HIF-1α levels persist even after weeks of hypoxia (95). Hypoxia-induced HIF-1α expression is less pronounced in aged (24 mo old) compared with young (3 mo old) rats (66), which is consistent with studies of limb ischemia in aged mice (23).
Sensory information from the carotid body is integrated in neurons of the commissural nucleus of the nucleus tractus solitarius (NTS) (86, 121, 229). Continuous hypoxia increases HIF-1 expression in the NTS, but not in the area prostrema, which is adjacent to it (252). These observations suggest that hypoxia selectively increases HIF-1 expression in neurons associated with the chemosensory reflex pathway.
3. HIF-1 target genes and ventilatory adaptation to chronic hypoxia
Chronic hypoxia alters the carotid body morphology, augments the sensory response to hypoxia, and affects the neurotransmitters/modulators in the chemosensory reflex pathway (19, 266). Many HIF-1 target genes are implicated in chronic hypoxia-induced changes in morphology and physiology of the chemoreflex pathway, as discussed below.
A) VEGF
Chronic hypoxia leads to hypertrophy of the carotid body, neovascularization, and hyperplasia of glomus cells (65, 111, 147, 177, 258). VEGF has been implicated in chronic hypoxia-induced changes in the morphology of the carotid body (147).
B) EPO
Circulating levels of Epo are elevated during chronic hypoxia. A recent study suggested that in addition to increasing O2 delivery via erythropoiesis, Epo also stimulates the carotid body and reflex stimulation of breathing (314). Studies on mice with targeted overexpression of Epo in the brain showed an enhancement of ventilatory acclimatization, which was attributed to altered catecholamine metabolism in brain stem neurons associated within the chemosensory reflex pathway (314).
C) ENDOTHELIN-1
Endothelin-1 (ET-1), a vasoactive peptide, is expressed at low levels in glomus cells of the carotid body, and chronic hypoxia markedly upregulates its expression (45). HIF-1 also mediates increased ET-1 expression in response to hypoxia in other cell types (124). Chronic hypoxia-induced sensitization of the hypoxic response and changes in carotid body morphology are blocked by ETA receptor antagonists (45, 46). The hypoxic ventilatory response is blunted in mice with heterozygous deletion of the gene encoding pre-pro-ET-1 (174).
D) OTHER HIF-1 TARGET GENES
HIF-1 mediates transcriptional activation of the genes encoding heme oxygenase-1 (HO-1) (188), inducible nitric oxide synthase (iNOS) (226), and angiotensin converting enzyme (ACE) (381). Hypoxia-induced neuronal NOS (nNOS) transcription is also shown to be mediated by HIF-1 (346). The expression of iNOS (366), nNOS (67), and ACE (178) is elevated in the carotid bodies of animals exposed to chronic hypoxia. Chronic hypoxia also leads to increased expression of nNOS (268) and HO-1 in the nervous system (222). However, the role of increased expression of these enzymes in ventilatory adaptation to chronic hypoxia has not been established.
4. Chronic hypoxia and HIF-2α expression in the chemosensory reflex pathway
Continuous hypoxia also increases HIF-2α accumulation primarily in glomus cells (179, 283). Relatively little is known about the effects of chronic hypoxia on HIF-2α expression in the chemosensory reflex pathway and its role in ventilatory adaptation to chronic hypoxia.
5. HIFs and evolutionary adaptation in high-altitude populations
Tibetan and Andean populations have resided at high altitude for thousands of years and have adapted to their hypoxic environment differently. Tibetans have developed increased ventilatory capacity, whereas Andeans have higher hemoglobin concentrations than lowlanders (10). The Tibetans are believed to have a longer history at high altitude, and the absence of polycythemia, which is a risk factor for stroke and perinatal mortality, suggests that the Tibetans have adapted more effectively to life in low O2.
Independent studies from four different research groups reported a remarkable correlation between genetic variation involving HIF signaling pathways and physiological adaptation in Tibetan and Andean populations. A study by Bigham et al. (18) suggested that HIF-1 target genes play a role in high-altitude adaptations in Andeans. Simonson et al. (310) found a significant association of selected haplotypes at the EGLN1 and PPARA loci (which are HIF-1 target genes that encode prolyl hydroxylase PHD2 and peroxisome proliferator associated receptor-α, respectively) with nonelevated hemoglobin phenotype that is unique to Tibetans. Genome-wide analysis of Tibetans by two independent groups revealed a high correlation between the presence of single-nucleotide polymorphisms at the EPAS1 locus (which encodes HIF-2α) and nonelevated hemoglobin concentrations in this population (11, 367). These studies on human populations, when taken together with the experimental models described above, provide compelling evidence that HIF signaling plays a major role in evolutionary and physiological adaptations to chronic hypoxia.
VI. NEONATAL ADAPTATION: ADRENAL MEDULLARY RESPONSES TO HYPOXIA
A. Adaptive Responses of Neonatal Adrenal Chromaffin Cells to Hypoxia
Neonates often experience umbilical cord compression and hypoxia during birth, which triggers a series of adaptive responses, including surfactant secretion and fluid absorption in the lungs, which are critical for facilitating air breathing (295) as well as increased glucose utilization by brain and stimulation of carbohydrate metabolism (176).
These physiological adaptations critically depend on hypoxia-induced catecholamine secretion from chromaffin cells in the adrenal medulla. Like glomus cells of the carotid body, neonatal (but not adult) adrenal chromaffin cells are extremely sensitive to hypoxia and secrete catecholamines within seconds after the onset of reduced O2 levels (25).
Nicotine use by pregnant mothers is correlated with higher incidence of premature delivery and sudden infant death syndrome, and these adverse effects were attributed in part to impaired hypoxia-evoked catecholamine secretion from adrenal chromaffin cells (55, 312). Pups born to nicotine-treated pregnant rats exhibit selective impairment of adrenal chromaffin cell responses to hypoxia and a high mortality rate (32, 33). Silencing HIF-2α function by short hairpin RNA completely prevented nicotine-induced loss of hypoxic sensitivity of adrenal chromaffin cells (32). Although the underlying mechanisms have not been elucidated, these observations suggest that HIF-2α is required for nicotine-induced loss of neonatal chromaffin cell responses to hypoxia, which is of considerable clinical and physiological significance.
B. Chronic Hypoxia and Catecholamine Synthesis
Besides the sympathetic nervous system, adrenal medullary chromaffin cells are the major source of catecholamines in the peripheral nervous system. In response to stress, chromaffin cells secrete catecholamines, which in turn initiate a variety of physiological adaptations to overcome the stress, a phenomenon referred as the “fight-or-flight” response. Recent studies suggest that HIFs play critical roles in the regulation of catecholamine biosynthesis under basal as well as chronic hypoxic conditions.
1. Basal catecholamine synthesis
HIF-2α expression is relatively high in adrenal chromaffin cells, and HIF-2α knockout mice exhibit markedly decreased levels of noradrenaline (NA) with high mortality at midgestation, but supplementation with the NA precursor l-threo-3,4-dihydroxyphenylserine (DOPS) improves survival (330). Loss of HIF-2α function in MAH cells (v-myc, adrenal-derived, HNK cell line derived from fetal sympathoadrenal progenitor cells) results in a dramatic reduction in NA levels associated with reduced expression of DOPA decarboxylase (DDC) and dopamine β-hydroxylase, which are key enzymes required for NA biosynthesis (26). These studies suggest that HIF signaling regulates basal catecholamine synthesis in adrenal chromaffin cells. Mice with targeted deletion of prolyl hydroxylase PHD3 exhibit impaired adrenal medullary secretion and sympathoadrenal responses to stress as well as decreased blood pressure (20). It was proposed that PHD3 and HIF-2α regulate development of the sympathoadrenal system. Taken together, these studies suggest that HIF signaling may impact on both developmental and physiological regulation of the sympathoadrenal system.
2. Chronic hypoxia and catecholamine synthesis
Chronic hypoxia upregulates phenylethanolamine N-methyltransferase (PNMT) gene expression in adrenal chromaffin cells (82) and in PC12 pheochromocytoma cells (323). PNMT encodes the enzyme critical for synthesis of epinephrine. HIF-1 mediates hypoxia-induced PNMT expression in PC12 cells via upregulation of Egr-1 and Sp-1, and it was proposed that HIF-1α serves as an “on-off” switch for hypoxic regulation of epinephrine synthesis via PNMT (355).
3. Chronic hypoxia: metabolic changes and ion channel expression in chromaffin cells
Mammalian cellular adaptations to chronic hypoxia involve reprogramming of mitochondrial metabolism involving activation of genes encoding PDK1 and COX4–2, which reduce ROS generation, as discussed in section III above. MAH cells subjected to chronic hypoxia exhibit increased PDK1 and COX4–2 mRNA expression, whereas the expression of other COX subunits such as COX5a and COX15 were unchanged (25).
Chronic hypoxia increases HIF-2α protein levels in MAH cells, and this response requires a functional mitochondrial ETC, with redistribution of cellular O2 gradients rather than increased mitochondrial ROS generation being reported (27). The Cav3.2 subtype of T-type Ca2+ channel facilitates exocytosis in adrenal chromaffin cells. Chronic hypoxia upregulates the expression of Cav3.2 channels in adrenal chromaffin cells (39) and in PC12 cells (62), and this response requires HIF-2α. Thus HIF signaling contributes to catecholamine synthesis, metabolic reprogramming, and Ca2+ channel regulation in adrenal chromaffin cells in response to chronic hypoxia.
VII. SYSTEMIC VASCULAR RESPONSES TO TISSUE HYPOXIA-ISCHEMIA
A. Hypoxia Induces Angiogenic Growth Factor Expression
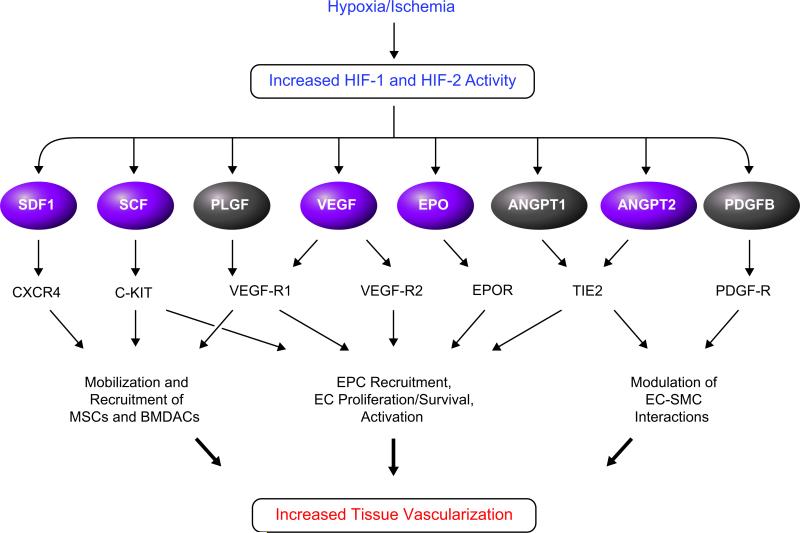
When cells do not receive adequate perfusion, local tissue hypoxia induces the accumulation of HIF-1α and HIF-2α protein, as described in section II. HIF-1 and HIF-2 activate the transcription of multiple genes encoding angiogenic growth factors and cytokines (FIGURE 9), including VEGF, angiopoietin (ANGPT) 1 and 2, placental growth factor (PLGF), platelet-derived growth factor B (PDGF-B), Epo, stromal-derived factor 1 (SDF-1), and stem cell factor (SCF) (23, 41, 70, 90, 153, 211, 234, 309). Of these, HIF-1 has been shown to directly bind to HREs in the promoters of the genes encoding VEGF (90), SDF-1 (41), ANGPT2 (309), and SCF (23), whereas the molecular mechanisms by which HIF-1 regulates expression of ANGPT1, PDGF-B, and PLGF mRNA remain to be determined. ANGPT2 gene expression is also regulated by ETS-1 (309), expression of which is HIF-1-regulated (245), indicating that ANGPT2 expression is both directly and indirectly regulated by HIF-1. Whereas VEGF gene transcription is activated when most cells are exposed to hypoxia, the other angiogenic factors are activated only in certain cell types. Expression of the ANGPT1 and ANGPT2 genes can be activated or repressed by hypoxia, depending on the cell type, and in each case the response is HIF-1 dependent (153).
FIGURE 9.
HIF-dependent production of angiogenic growth factors and cytokines in hypoxic cells. Genes encoding the factors shown in purple are directly activated by HIF-1.
Multiple vascular cell types, most notably endothelial cells, are targets for stimulation by an angiogenic growth factor such as VEGF because they express the cognate receptor (VEGFR1 or VEGFR2) (FIGURE 9). Many angiogenic factors, including PLGF, SDF1, and VEGF, also function as cytokines that recruit proangiogenic cells from distant sites such as the bone marrow (105, 146, 200). In addition to the effects induced by ligand-receptor coupling, hypoxia has direct, cell-autonomous effects on endothelial cells that are mediated by HIF-1 (38, 194, 211, 326) and HIF-2 (311). Remarkably, several of the receptors for angiogenic factors are also regulated by HIF-1 in specific proangiogenic cell types, such as the expression of VEGFR1 in mesenchymal stem cells (246). Thus HIF-1 functions as a master regulator of angiogenesis by activating the expression of multiple genes in hypoxic-ischemic cells that send out angiogenic signals as well as in the cells that respond to those signals. The result is an increase in the capillary density and tissue perfusion, an increase in tissue oxygenation, and elimination of the hypoxic stimulus.
B. Increased O2 Consumption Stimulates Angiogenesis
An increase in tissue mass, resulting from hyperplasia (increase in cell number) and/or hypertrophy (increase in cell size), is usually associated with an increase in O2 consumption. For example, individuals with systemic hypertension develop left ventricular hypertrophy in order for the heart to overcome the increased resistance to blood flow and maintain cardiac output. Relative to many other organs, the heart generates a greater fraction of its ATP from oxidative phosphorylation. Cardiac hypertrophy leads to decreased tissue oxygenation as a result of increased O2 consumption. In a mouse model of pressure-induced left ventricular hypertrophy due to transaortic constriction, myocardial hypertrophy was associated with increased myocardial HIF-1α protein and VEGF mRNA levels (287). HIF-1-dependent activation of the genes encoding VEGF and other angiogenic growth factors leads to increased angiogenesis, which is the sprouting of new capillaries from existing vessels. The increased O2 delivery associated with perfusion through the new blood vessels compensates for the increased O2 consumption, returning the heart tissue to a normoxic state (287).
Hypoxia can also result if a constant number of cells increase their O2 consumption. Chronic anemia induces increased cardiac output to compensate for reduced blood O2-carrying capacity, and the increased O2 consumption associated with increased cardiac work also dramatically increases myocardial HIF-1α protein, VEGF mRNA and protein levels, and myocardial capillary density (215).
C. Occlusion of a Major Artery Stimulates Arteriogenesis
Stenosis of a major coronary or peripheral artery as a result of atherosclerosis leads to a reduction in blood flow distal to the occlusion. Whereas collateral blood vessels may be able to supply sufficient O2 to the tissue under basal conditions, there is little reserve available to increase blood flow in response to increased O2 consumption, resulting in tissue hypoxia/ischemia and onset of symptoms. Critical stenosis represents a degree of blood flow reduction that results in symptoms even at rest and will lead to tissue necrosis in the absence of treatment. The adaptive physiological response to arterial stenosis is to increase the luminal area of collateral blood vessels, a process referred to as collateralization or arteriogenesis (5). It is important to emphasize that, in contrast to angiogenesis, this process does not involve new blood vessel formation but rather denotes the remodeling of existingvesselstoaccommodateincreasedbloodflowthroughan increase in luminal diameter.
Although hypoxia does not occur in the immediate region of the collateral remodeling, it does occur in the distal vascular bed where angiogenesis is also stimulated (195). Expression of monocyte chemoattractant protein 1 is highly induced in ischemic calf muscle in mice subjected to femoral artery ligation (23), yet it plays an important role in the recovery of perfusion by remodeling of collateral vessels present in the thigh (338, 342). Angiogenic factors such as VEGF and PLGF also stimulate the remodeling of collateral blood vessels following arterial ligation (54, 261). Since the restriction of blood flow through conduit vessels results in decreased perfusion in the downstream vascular bed, it is not surprising that signals from the ischemic tissue play critical roles in the remodeling of collateral vessels. In the standard experimental model of arterial stenosis involving unilateral ligation of the femoral artery, Hif-1α+/− mice show impaired recovery of perfusion relative to wild-type littermates at all ages and suffer more severe ischemic tissue damage with aging, associated with impaired expression of HIF-1α protein and of mRNAs encoding the angiogenic factors ANGPT1, ANGPT2, PLGF, SCF, SDF1, and VEGF in the ischemic limb (23, 278). Ischemia-induced mobilization of CD34+VEGFR2+ and Sca1+CXCR4+ bone marrow-derived angiogenic cells is also impaired in Hif-1α+/− mice, which is due at least in part to reduced expression in the ischemic muscle of VEGF and SDF-1, which are the ligands for VEGFR2 and CXCR4, respectively (23).
VIII. PULMONARY VASCULAR RESPONSES TO ALVEOLAR HYPOXIA
A. Acute Response: Hypoxic Pulmonary Vasoconstriction
Arterioles in the systemic circulation dilate in response to hypoxia to increase tissue perfusion and O2 delivery. In contrast, pulmonary arterioles constrict in response to hypoxia to shunt blood away from regions of the lung that are not ventilated, as in the case of pneumonia, in which a pulmonary lobule can be infiltrated with bacteria, leukocytes, and fluid that obliterate the alveolar air spaces. Thus matching of ventilation and perfusion is an adaptive mechanism to maximize gas exchange and blood oxygenation. However, in patients with chronic lung disease, hypoxia is widespread throughout the lungs, and the resulting hypoxic pulmonary vasoconstriction (HPV) further reduces O2 uptake. In addition, increased pulmonary tone increases pulmonary vascular resistance, leading to right ventricular hypertrophy. Residence at high altitude also results in pan-alveolar hypoxia that can trigger pulmonary hypertension in susceptible individuals.
Although there is a general consensus that the response of PASMCs plays a critical role in HPV, the precise molecular mechanisms leading to PASMC depolarization and contraction have not been fully delineated (56). The increase in PASMC intracellular calcium concentration ([Ca2+]i) that is induced by acute hypoxia appears to result from the release of Ca2+ from the sarcoplasmic reticulum (71, 233). Hypoxia inhibits opening of voltage-gated K+ channels Kv1.5 and Kv2.1 (3), which contributes to PASMC depolarization. Hypoxia also sensitizes PASMCs to increased [Ca2+]i, an effect that is mediated by RhoA/Rho kinase signaling, which inhibits the dephosphorylation of myosin light chain, thereby promoting PASMC contraction (279). It is not known whether pulmonary arterial responses to acute hypoxia are dependent on HIF activity, as has been reported for the carotid body (159), although the finding of increased HPV in individuals with CP (313) supports this hypothesis.
B. Chronic Response: Pulmonary Arterial Hypertension
Exposure to chronic hypoxia induces functional and structural changes in the endothelial cells, smooth muscle cells, and fibroblasts that make up the intima, media, and adventitia of pulmonary arterial vessels, respectively, and these changes lead to the development of pulmonary arterial hypertension (PAH) (235). The specific vascular remodeling that is induced by hypoxia is complex and variable, based on species, sex, and age of the host as well as varying among vessels at different sites along the longitudinal axis of the pulmonary vascular tree (319).
1. Fibroblasts
When calves are subjected to chronic hypoxia, dramatic hypertrophy of the medial layer of the pulmonary arterioles occurs (91). Medial hypertrophy is accompanied by expansion of the vasa vasorum, which is the system of blood vessels that perfuse the vessel wall itself (356). Given the major role of HIFs in ischemic-induced vascularization that is described above in section VII and the major role of HIF-1 in pulmonary artery smooth muscle cells that is described below in section VIIIB3, it is likely that HIFs contribute to the expansion of vasa vasorum in pulmonary arterioles of calves subjected to chronic hypoxia, although it is difficult to obtain mechanistic data in a large animal model. Suprarenal aortic constriction in rats induced expansion in the adventitial vasa vasorum of the aorta that was temporally associated with activation of HIF-1 and VEGF signaling (173).
2. Smooth muscle cells
Exposure to acute hypoxia causes an increase in [Ca2+]i that is reversible upon reoxygenation. In contrast, in PASMCs subjected to chronic hypoxia, [Ca2+]i remains elevated even after return to normoxia, an effect that requires extracellular Ca2+ (307). This effect is mediated by store-operated Ca2+ channels, which are activated by depletion of intracellular Ca2+ stores during chronic hypoxia, and Ca2+ influx through these channels is responsible for the elevated baseline [Ca2+]i in PASMCs isolated from rats subjected to chronic hypoxia (345). These channels are composed of transient receptor potential (TRP) proteins, TRPC1 and TRPC6 mRNA and protein expression was increased in PASMCs from hypoxic rats or in PASMCs cultured under hypoxic conditions for 60 h, demonstrating that the response is cell autonomous (345). TRPC1 and TRPC6 mRNA and protein levels were also increased in PASMCs from WT mice subjected to chronic hypoxia, whereas chronic hypoxia did not increase baseline [Ca2+]i or TRPC1/TRPC6 expression in PASMCs of Hif-1α+/− mice (306, 345). Infection of PASMCs with AdCA5, which is an adenovirus encoding a constitutively active form of HIF-1α, increased TRPC1 and TRPC6 expression under nonhypoxic conditions. Thus both gain- and loss-of-function studies implicate HIF-1 in the regulation of [Ca2+]i.
In contrast to the increased TRPC1/TRPC6 expression, Kv1.5 and Kv2.1 mRNA and protein expression are decreased in PASMCs subjected to chronic hypoxia in vivo or ex vivo (276, 375), and these changes in gene expression are also HIF-1α dependent (22, 350). As discussed in section II above, HIF-1 is a transcriptional activator, and its repression of Kv1.5 and Kv2.1 appears to be an indirect/secondary effect of HIF-1-dependent induction of ET-1 expression. Whereas PASMCs from WT mice subjected to chronic hypoxia show inhibition of K+ currents and depolarization, these responses are absent in PASMCs from Hif-1α+/− mice (306). Thus, whereas the response to acute hypoxia (time scale: seconds) involves the inactivation of Kv channels, the response to chronic hypoxia (time scale: days) involves a reduction in the steady-state levels of the channel proteins (FIGURE 10).
FIGURE 10.
HIF-1-dependent responses to hypoxia in pulmonary artery smooth muscle cells.
In addition to increased [Ca2+]i, chronic hypoxia also results in increased intracellular pH (pHi) in PASMCs, an effect that is due to HIF-1-dependent expression of the sodium-hydrogen exchanger NHE1 (305). Increased [Ca2+]i and pHi contribute to the activation of signal transduction pathways that promote PASMC hypertrophy and hyperplasia, which leads to the medial thickening of pulmonary arterioles that is a pathological hallmark of hypoxia-induced PAH. Exposure of WT but not Hif-1α+/− mice to chronic hypoxia induces PASMC alkalinization and hypertrophy (305, 306). On the basis of all of the HIF-1-dependent changes in PASMC physiology, which are described above and summarized in FIGURE 10, it is not surprising that the development of hypoxia-induced pulmonary arteriole muscularization, PAH, and right ventricular hypertrophy, which is observed when WT mice are exposed to 10% O2 (70 mmHg) for 3 wk, is significantly impaired in Hif-1α+/− mice (306, 370).
The demonstration that mice partially deficient in HIF-1α expression are protected against the development of PAH suggests that pharmacological inhibition of HIF-1 may be of therapeutic benefit in this clinical context. This hypothesis was tested by the daily administration of digoxin, a cardiac glycoside that has been used to treat congestive heart failure and cardiac arrythmias for decades, which inhibits the synthesis of HIF-1α protein (379). Treatment with digoxin attenuated the development of right ventricular hypertrophy and prevented the changes in pulmonary vascular [Ca2+]i, pHi, remodeling, and pressure that occur in mice exposed to chronic hypoxia (1).
The role of HIF-1 in the pathogenesis of PAH is not restricted to hypoxia-induced PAH. The spontaneous development of PAH in fawn-hooded rats is associated with increased HIF-1α expression, HIF-dependent reductions in Kv currents and Kv1.5 expression, increased PDK1 expression, and a switch from oxidative to glycolytic metabolism in pulmonary artery smooth muscle cells (22). These metabolic changes appear to play a critical role in the pathogenesis of PAH because treatment of animals with dichloroacetate, an inhibitor of PDK1, leads to regression of PAH (228). A feed-forward mechanism (see sect. IIF) may be involved because the administration of dichloroacetate normalizes HIF-1α and Kv1.5 expression (22). It should be noted in this regard that PDK1 blocks the conversion of pyruvate to AcCoA (see sect. III), and pyruvate has been shown to inhibit the prolyl hydroxylation and degradation of HIF-1α (201).
3. Endothelial cells
In the most severe form of PAH, endothelial cells undergo dysregulated proliferation to generate plexiform lesions that may totally occlude the vessel. Whereas endothelial cells normally express VEGFR2 and respond to VEGF produced by other cell types, the endothelial cells of plexiform lesions were found to express high levels of VEGF in association with high levels of HIF-1α and HIF-1β, as determined by immunohistochemical analysis of lung biopsies from PAH patients, suggesting the activation of an autocrine signaling pathway similar to what is observed in tumor cells (335). The molecular basis for the increased HIF-1 and VEGF signaling in the endothelial cells of plexiform lesions has not been determined. Remarkably, HIF-1-dependent reprogramming from oxidative to glycolytic metabolism also occurs in endothelial cells of patients with idiopathic (i.e., not hypoxia-induced) PAH and is stably maintained even when the cells are passaged under standard tissue culture conditions of 20% O2 (84).
As described above for Hif-1α+/− mice, Hif-2α+/− mice also have attenuated development of hypoxia-induced PAH (30). Pulmonary ET-1 and plasma catecholamine levels were elevated in chronically hypoxic WT, but not Hif-2α+/−, mice. ET-1 induces changes in PASMCs, such as decreased Kv channel expression (350), Rho kinase-mediated Ca2+ sensitization (119), and cell hypertrophy (64). Furthermore, ET-1 receptor antagonists inhibit the development of hypoxia-induced PAH (247). Surprisingly, however, mice with conditional knockout of HIF-2α in vascular endothelial cells (due to expression of Cre recombinase from the promoter of the VE-cadherin gene) developed spontaneous PAH under ambient room-air conditions, which was associated with increased permeability of lung blood vessels (311). These results suggest the existence of complex and poorly understood functional interactions between HIF-1α and HIF-2α in pulmonary vascular endothelial and smooth muscle cells.
IX. ERYTHROPOIESIS
The major acute and chronic mechanisms by which mammals sense systemic hypoxia are via the carotid body, which triggers cardiovascular and respiratory responses (as described in section V), and via EPO-producing cells in the kidney and liver, which trigger augmented erythropoiesis that eventually increases the blood O2-carrying capacity. As described for the vascular response to hypoxia (see sect. VII), HIFs function as master regulators of erythropoiesis by controlling the expression not only of the genes encoding EPO (303) and its receptor EPOR (211, 368), but also a battery of genes that regulate intestinal uptake, transport, and bone marrow utilization of iron, which is required for the synthesis of hemoglobin and its function as an O2 carrier (FIGURE 11). Thus HIFs repress transcription of the gene encoding hepcidin (260) and activate transcription of the genes encoding transferrin (281), transferrin receptor (199, 322), and divalent metal transporter 1 (217). HIF-2α appears to play a key role in regulating erythropoiesis in adult mice (104, 293, 347), although HIF-1α is also required for yolk sac erythropoiesis (368), renal EPO production in response to acute hypoxic episodes (34), and the development of polycythemia in response to chronic hypoxia (370).
FIGURE 11.
HIF-dependent regulation of iron homeostasis and erthropoiesis.
X. MALADAPTIVE RESPONSES TO INTERMITTENT HYPOXIA
A. Pathophysiology of Sleep-Disordered Breathing With Recurrent Apnea
Although various forms of intermittent hypoxia may be encountered in life (264), the following section focuses on the effects of intermittent hypoxia in the context of recurrent apneas associated with sleep-disordered breathing, which is a major cause of morbidity and mortality affecting several million Americans. In this condition, transient repetitive cessation of breathing (i.e., apnea) lasting 10–40 s results in periodic hypoxemia (decreased Po2 in arterial blood). The frequency of apnea may exceed 60 episodes per hour in severely affected patients, and O2 saturation of blood hemoglobin may be reduced to as low as 50%. Recurrent apnea patients are prone to develop systemic hypertension (241, 304, 315) and exhibit elevated sympathetic nerve activity, enhanced sympathetic nerve response to acute hypoxia, as well as elevated plasma and urinary catecholamines (4, 97, 151).
Although recurrent apneas result in both chronic intermittent hypoxia (CIH) and chronic intermittent hypercapnia, exposure of rodents to ambient CIH alone was sufficient to induce cardiovascular changes similar to those seen in recurrent apnea patients (88). Rats and mice exposed to 10 days of CIH, consisting of alternating cycles of hypoxia [5% O2 (35 mmHg) for 15 s] and normoxia [21% O2 (147 mmHg) for 5 min], with 9 episodes/h for 8 h/day, exhibit elevated plasma catecholamines levels and increased mean blood pressure (171, 257). The carotid bodies constitute the frontline defense system for detecting systemic hypoxia associated with apnea (53, 267). CIH exerts two major effects on the chemoreceptor reflex pathway: 1) augmented carotid body response to acute hypoxia (256, 267, 277) and 2) long-lasting activation of the carotid body that persists for several hours after the termination of CIH, a phenomenon referred as sensory long-term facilitation (255). Studies involving human subjects (239) and experimental animals (191) suggest that elevated sympathetic nerve activity in CIH is due to enhanced carotid body chemoreceptor activity. In addition to the sympathetic nervous system, increased catecholamine secretion from the adrenal medulla also contributes to elevated blood pressure and plasma catecholamine levels (8). CIH selectively facilitates catecholamine secretion from the adrenal medulla in response to hypoxia (171, 316, 317) but not in response to hypercapnia (171) or other depolarizing stimuli (316).
B. Oxidants Mediate Cardiovascular Pathology in Response to CIH
It was proposed that increased generation of ROS during hypoxia-reoxygenation mediates the pathological effects of CIH (264). Indeed, ROS levels were elevated in the carotid body (255), adrenal medulla (171, 316), and central nervous system (257, 273, 377) of rats and mice exposed to CIH. Recurrent apnea patients also exhibit elevated ROS levels (4). Remarkably, the effects of CIH on catecholamine secretion, blood pressure elevation, long-term facilitation of respiratory motor activity, and sensory long-term facilitation in the carotid body can all be blocked by concurrent treatment of animals with the superoxide scavenger MnTMPyP (171, 255, 257, 265, 334). Obstructive sleep apnea patients exhibit impaired vasodilation, and antioxidant treatment restores the vascular responses (103). These studies suggest that ROS play a critical role in mediating the cardiovascular pathology associated with CIH.
C. Role of HIF-1 and HIF-2 in Causing Oxidative Stress in CIH
1. Intermittent hypoxia increases HIF-1 activity
The effect of CIH on rat PC12 adrenal pheochromocytoma cells has been modeled in an incubator chamber in which the O2 concentration is rapidly decreased from 20% O2 (140 mmHg) to 1.5% O2 (10.5 mmHg) for 30 s followed by reoxygenation for 4 min at 20% O2. HIF-1α protein levels and HIF-1 transcriptional activity increased progressively as the number of cycles of hypoxia-reoxygenation increased from 10 to 30 to 60 to 120 (373). Remarkably, CIH induced HIF-1 activity via multiple signal transduction pathways, which were all activated by an increase in ROS that was dependent on NADPH oxidase (Nox) activity (374). HIF-1α protein levels were induced by protein kinase C (PKC)-dependent activation of mTOR, leading to increased translation of HIF-1α mRNA into protein (as described in sect. IIE) and PKC-dependent inhibition of prolyl hydroxylase activity, leading to decreased degradation of HIF-1α protein (374). In addition, Ca2+/calmodulin kinase (CaMK) activity was induced by CIH in these cells, leading to phosphorylation of p300 and increased association with HIF-1α, thereby stimulating HIF-1α transactivation function (373). The induction of PKC and CaMK activity in cells exposed to CIH is dependent on the activation by ROS of phospholipase C-γ, which breaks down phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (373, 374). IP3 receptor activation then leads to increased intracellular Ca2+ levels, providing all of the necessary signals for activation of PKC and CaMK (FIGURE 12). In contrast to continous hypoxia, in which reoxygenation leads to a rapid decay (t1/2 <5 min) in the levels of HIF-1α (343), HIF-1α levels remain persistently elevated (t1/2 >90 min) following CIH, due to continued mTOR activity (374).
FIGURE 12.
Induction of HIF-1 activity by intermittent hypoxia. IH increases the levels of reactive oxygen species (ROS), which activate phospholipase C-γ which converts phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), resulting in increased intracellular concentration of Ca2+ ([Ca2+]i) and subsequent activation of protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase (CaMK). PKC inhibits PHD2 activity, leading to increased HIF-1α protein stability, and stimulates mammalian target of rapamycin (mTOR) activity, leading to increased HIF-1α protein synthesis. CaMK stimulates the binding of p300 to HIF-1α, leading to increased HIF-1-dependent transcriptional activity.
2. HIF-1 mediates CIH-induced Nox expression: a feed-forward mechanism
CIH increases Nox activity, which is a major cellular source of ROS (13) in the carotid body (254), adrenal medulla (317), and central nervous system (377). Of the various Nox isoforms, Nox2 has been implicated in mediating the effects of CIH on carotid body function (254), catecholamine secretion from adrenal medulla (317), and sleep behavior (377). CIH upregulates Nox2 mRNA, protein, and enzyme activity in PC12 cells and WT MEFs, and these effects were abolished or attenuated by blocking HIF-1 activity through RNA interference, pharmacological inhibition (using digoxin or YC-1), or by genetic KO of HIF-1α in MEFs (372).
Nox2 mRNA and activity were also upregulated in the carotid body, brain stem, and cerebral cortex in WT but not in Hif-1α+/− mice exposed to CIH (372). Hif-1α+/− mice exposed to CIH exhibit striking absence of oxidative stress and autonomic abnormalities that are observed in WT littermates including the absence of heightened carotid body activity, elevated plasma catecholamines, exaggerated hypoxic ventilatory response, and hypertension (257). Recurrent apnea patients exhibit hypercholesterolemia and hypertriglyceridemia, and these responses were also observed in WT mice exposed to CIH for 5 days, whereas the development of hypertriglyceridemia was blunted in CIH-exposed Hif-1α+/− mice (193). Interestingly, HIF-1 activation in response to CIH requires Nox2 expression (374). In turn, HIF-1 mediates activation of Nox2 expression (372). This feed-forward mechanism appears to play a critical role in generating the persistent oxidative stress associated with CIH. Moreover, the pro-oxidant role of HIF-1 in the maladaptive (pathological) setting of CIH stands in contrast to the antioxidant role of HIF-1 in the adaptive (physiological) response to continuous hypoxia, wherein HIF-1 mediates the expression of multiple pathways that modulate or decrease mitochondrial respiration and ROS generation as discussed in section III.
3. Intermittent hypoxia downregulates HIF-2α protein expression
In striking contrast to increased HIF-1α expression described above, CIH decreases HIF-2α protein expression in rat PC12 cells in a stimulus-dependent manner (238). The effects of CIH were long lasting as evidenced by the delayed return of HIF-2α protein levels until 16 h after terminating CIH. Similar decreases in HIF-2α by CIH were also seen in carotid bodies and adrenal medullae from CIH-exposed rats (238) and in MAH cells (25). Inhibitors of Ca2+-activated proteases (calpains) prevented the decline in HIF-2α protein levels in CIH-exposed PC12 cells, whereas inhibitors of prolyl hydroxylases or the proteasome were ineffective, suggesting that calpains specifically mediate HIF-2α degradation in response to CIH. Involvement of calpains was further supported by the finding that CIH caused persistent elevation of basal cytosolic Ca2+ and increased calpain activity (238). Thus Ca2+-dependent proteases mediate HIF-2α degradation, whereas Ca2+-dependent activation of phospholipase C-γ, PKC, and mTOR contribute to HIF-1α accumulation in response to CIH, indicating that ROS-dependent Ca2+ signaling plays a critical role in causing HIF-1α and HIF-2α imbalance (FIGURE 13).
FIGURE 13.
Differential regulation of HIF-1α and HIF-2α by chronic intermittent hypoxia (CIH) contributes to oxidative stress and cardiorespiratory pathology. CIH increases reactive oxygen species (ROS) via NADPH oxidase-2 (NOX-2) activation. ROS elevate cytosolic calcium [Ca2+]i. Elevated [Ca2+]i leads to increased HIF-1α protein levels as a result of Ca2+-dependent protein kinase C (PKC) activation of mTOR and inhibition of PHDs. Ca2+-activated proteases (calpains) mediate HIF-2α degradation by CIH. HIF-1 activates transcription of the gene encoding the pro-oxidant enzyme NOX-2, whereas decreased HIF-2 activity leads to decreased transcription of genes encoding antioxidant enzymes such as superoxide dismutase 2 (SOD-2). The imbalance between pro-oxidant and anti-oxidant enzymes results in oxidative stress and cardiorespiratory pathology.
4. HIF-2α downregulation contributes to CIH-induced oxidative stress
Oxidative stress may arise due to increased generation of ROS by pro-oxidants, such as Nox2, or due to decreased activity of antioxidant enzymes, such as superoxide dismutase (SOD). HIF-2 is a potent transactivator of several genes encoding antioxidant enzymes, including copper/zinc and manganese SOD (which are encoded by Sod1 and Sod2, respectively), catalase, and glutathione peroxidase 1 (292). CIH-induced oxidative stress was associated with decreased Sod2 promoter activity and reduced SOD2 enzyme activity, and these effects were prevented by transfection of a HIF-2α expression vector into PC12 cells (238). Systemic administration of a calpain inhibitor restored HIF-2α levels and SOD2 activity and prevented oxidative stress, hypertension, and elevated plasma catecholamine levels in CIH-exposed rats (238). Hif-2α+/− mice when maintained under normal room air conditions exhibit markedly reduced levels of mRNAs encoding SOD2, SOD1, and catalase in the carotid body and adrenal medulla; oxidative stress; augmented carotid body sensitivity to hypoxia; irregular breathing and apneas; hypertension; and elevated plasma norepinephrine levels, which is a phenotype that is remarkably similar to that observed in WT mice exposed to CIH (253). These studies suggest that HIF-2α degradation contributes to CIH-induced oxidative stress via reduced levels of antioxidant enzymes. The consequences of the CIH-induced imbalance between HIF-1α and HIF-2α on redox status and cardiovascular function are summarized in FIGURE 13.
XI. SUMMARY AND PERSPECTIVE
The discovery of HIF-1 and subsequent identification of other members of the HIF family, notably HIF-2, has led to novel insights into the molecular basis of adaptive and maladaptive cellular and systemic responses to continuous and intermittent hypoxia. HIF-1α expression is conserved phylogenetically among metazoans from invertebrates to vertebrates, whereas HIF-2α appeared later in evolution in association with specialized systems of O2 delivery. The induction of HIFs by hypoxia is mediated by posttranslational changes (i.e., O2-dependent hydroxylation of proline and asparagine residues by PHD2 and FIH-1, respectively), which allow a rapid initiation (and termination) of transcriptional responses to reduced O2 availability. HIFs are also induced by other stimuli, including NO, ROS, growth factors, and cytokines. The repertoire of transcriptional responses to hypoxia mediated by HIFs is complex and involves an astounding variety of positive and negative feedback loops that operate in a context-dependent manner. The cellular and molecular complexity of HIF signaling provides enormous plasticity in the response to hypoxia, which is manifest over evolutionary and developmental time scales and across varied anatomical and physiological landscapes, and represents the basis of O2 homeostasis in all metazoan species.
While studies on cell culture models provided important insights into mechanisms of HIF activation by hypoxia, studies on experimental animals, as well as human subjects, have provided evidence for the role of HIFs in orchestrating (patho)physiological responses to acute and chronic hypoxia. Recent studies have provided compelling evidence in support of the surprising conclusion that HIFs are critical for carotid body responses to acute hypoxia. HIF-1α deficiency results in hyposensitivity, whereas HIF-2α deficiency induces hypersensitivity of the carotid body to hypoxia. The regulation of acute hypoxic sensing by HIFs has important physiological consequences. For instance, hyposensitivity to hypoxia impairs ventilatory acclimatization at high altitude, leading to more severe systemic hypoxia, which is similar to that seen in human subjects after carotid body resection (280). On the other hand, hypersensitivity of the carotid body to hypoxia causes unwarranted reflex activation of the sympathetic nervous system, resulting in cardiorespiratory pathology including systemic hypertension. In neonates, acute hypoxia evokes catecholamine secretion from adrenal chromaffin cells, which triggers physiological responses that are critical for transition from uterine to extrauterine life. In adults, various forms of stress, including acute hypoxia, induce the release of catecholamines from the adrenal medulla. Evidence is emerging suggesting important roles for HIFs in catecholamine synthesis and secretion.
Continuous hypoxia leads to a series of physiological adaptations that require activation of both HIF-1 and HIF-2, leading to altered expression of several hundred genes in any given cell. Some of the well-characterized HIF-mediated adaptations to continuous hypoxia include erythropoiesis and angiogenesis, which are critical for improved O2-carrying capacity and O2 transport to tissues. Another important adaptive response is ventilatory acclimatization to continuous hypoxia, which involves components of the peripheral and central nervous systems. Studies on mice with loss of function and humans with gain of function suggest that HIF-1 plays a critical role in mediating VAH. Recent studies of Tibetan and Andean populations have provided intriguing evidence suggesting that natural selection acting on genetic variation at loci encoding components of the HIF/hydroxylase system represents the molecular basis of inherited physiological adaptations to high-altitude hypoxia.
The HIF-1-mediated cellular adaptations to continuous hypoxia are of considerable physiological significance. At the cellular level, continuous hypoxia leads to reprogramming of mitochondrial metabolism that ensures adequate ATP generation and prevents adverse consequences of excess mitochondrial ROS generation. These metabolic adaptations are critical for cellular homeostasis and are due to HIF-1-mediated transcriptional regulation of glycolytic enzymes, mitochondrial ETC components, and other metabolic enzymes.
Unlike the many physiological adaptations to continuous hypoxia, CIH associated with recurrent apneas leads to maladaptation and cardiorespiratory pathology due to persistent oxidative stress. Cell culture and rodent models revealed that CIH causes activation of HIF-1α and downregulation of HIF-2α. The dysregulation of HIF-1 and HIF-2 contributes to further oxidative stress by increased HIF-1-dependent transcription of genes encoding pro-oxidant enzymes and decreased HIF-2-dependent transcription of genes encoding antioxidant enzymes. Taken together, these studies suggest that coordinated upregulation of HIF-1 and HIF-2 in response to continuous hypoxia mediates adaptive responses in the systemic circulation and maladaptive responses in the pulmonary circulation, whereas CIH triggers an imbalance between HIF-1 and HIF-2 activity that leads to oxidative stress, resulting in the maladaptive responses and cardiorespiratory pathology associated with CIH (FIGURE 14). This pathological state may also provide insight into the fundamental challenge of life with O2, which is to maintain the balance between the benefits of O2 in generating sufficient energy to sustain multitrillion-cell life forms and the dangers of O2 in generating toxic radicals capable of triggering cell death. We call this balance “oxygen homeostasis,” and it is the raison d'etre of the hypoxia-inducible factors.
FIGURE 14.
Consequences of responses to chronic continuous hypoxia and chronic intermittent hypoxia in pulmonary arterioles and carotid bodies, respectively.
ACKNOWLEDGMENTS
GRANTS
Research from authors’ laboratories is supported by National Institutes of Health Grants HL-90554, HL-76537, HL-86493 (to N. R. Prabhakar) and HHS-N268201000032C, P01-HL-65608, P20-GM-78494, R01-HL-55338, and U54-CA-143868 (to G. L. Semenza) as well as contracts/grants from the American Cancer Society, Japan Science and Technology Agency (ERATO-ICORP Gas Biology Project), and Johns Hopkins Institute for Cell Engineering (to G. L. Semenza).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hyper-tension in mice. Proc Natl Acad Sci USA. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006;66:5641–5647. doi: 10.1158/0008-5472.CAN-05-3345. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baby SM, Roy A, Mokashi AM, Lahiri S. Effects of hypoxia and intracellular iron chelation on hypoxia-inducible factor-1α and -1β in the rat carotid body and glomus cells. Histochem Cell Biol. 2003;120:343–352. doi: 10.1007/s00418-003-0588-2. [DOI] [PubMed] [Google Scholar]
- 7.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol. 1987;63:685–691. doi: 10.1152/jappl.1987.63.2.685. [DOI] [PubMed] [Google Scholar]
- 10.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck I, Ramirez S, Weinmann R, Caro J. Enhancer element at the 3′-flanking region controls transcriptional response to hypoxia in the human erythropoietin gene. J Biol Chem. 1991;266:15563–15566. [PubMed] [Google Scholar]
- 13.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 16.Beru N, McDonald J, Lacombe C, Goldwasser E. Expression of the erythropoietin gene. Mol Cell Biol. 1986;6:2571–2575. doi: 10.1128/mcb.6.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, Shriver MD. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics. 2009;4:79–90. doi: 10.1186/1479-7364-4-2-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisgard GE. Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol. 2000;121:237–246. doi: 10.1016/s0034-5687(00)00131-6. [DOI] [PubMed] [Google Scholar]
- 20.Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez-Juan A, Lopez-Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P, Ratcliffe PJ. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol. 2008;28:3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondurant MC, Koury MJ. Anemia induces accumulation of erythropoietin mRNA in the kidney and liver. Mol Cell Biol. 1986;6:2731–2733. doi: 10.1128/mcb.6.7.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrialhypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 23.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 24.Bracken CP, Fedele AO, Linke S, Balrak W, Lisy K, Whitelaw ML, Peet DJ. Cell-specific regulation of hypoxia-inducible factor (HIF)-1α and HIF-2α stabilization and transactivation in a graded oxygen environment. J Biol Chem. 2006;281:22575–22585. doi: 10.1074/jbc.M600288200. [DOI] [PubMed] [Google Scholar]
- 25.Brown ST, Buttigieg J, Nurse CA. Divergent roles of reactive oxygen species in the responses of perinatal adrenal chromaffin cells to hypoxic challenges. Respir Physiol Neurobiol. 2010;174:252–258. doi: 10.1016/j.resp.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Brown ST, Kelly KF, Daniel JM, Nurse CA. Hypoxia inducible factor (HIF)-2 α is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem. 2009;110:622–630. doi: 10.1111/j.1471-4159.2009.06153.x. [DOI] [PubMed] [Google Scholar]
- 27.Brown ST, Nurse CA. Induction of HIF-2α is dependent on mitochondrial O2 consumption in an O2-sensitive adrenomedullary chromaffin cell line. Am J Physiol Cell Physiol. 2008;294:C1305–C1312. doi: 10.1152/ajpcell.00007.2008. [DOI] [PubMed] [Google Scholar]
- 28.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 29.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111:1519–1527. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buerk DG. Measuring tissue PO2 with microelectrodes. Methods Enzymol. 2004;381:665–690. doi: 10.1016/S0076-6879(04)81043-7. [DOI] [PubMed] [Google Scholar]
- 32.Buttigieg J, Brown S, Holloway AC, Nurse CA. Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: role of α7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2α. J Neurosci. 2009;29:7137–7147. doi: 10.1523/JNEUROSCI.0544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buttigieg J, Brown S, Zhang M, Lowe M, Holloway AC, Nurse CA. Chronic nicotine in utero selectively suppresses hypoxic sensitivity in neonatal rat adrenal chromaffin cells. FASEB J. 2008;22:1317–1326. doi: 10.1096/fj.07-9194com. [DOI] [PubMed] [Google Scholar]
- 34.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–2053. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- 36.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 α. Cardiovasc Res. 2008;77:463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 38.Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1α-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107:2705–2712. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carabelli V, Marcantoni A, Comunanza V, de Luca A, Diaz J, Borges R, Carbone E. Chronic hypoxia up-regulates α1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol. 2007;584:149–165. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 41.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 42.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 α. Mol Cell Biol. 2005;25:6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1314–L1323. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1257–L1262. doi: 10.1152/ajplung.00419.2006. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem. 2011;286:13869–13878. doi: 10.1074/jbc.M110.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chilov D, Camenisch G, Kvietikova I, Ziegler U, Gassmann M, Wenger RH. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1α. J Cell Sci. 1999;112:1203–1212. doi: 10.1242/jcs.112.8.1203. [DOI] [PubMed] [Google Scholar]
- 49.Chua YL, Dufour E, Dassa EP, Rustin P, Jacobs HT, Taylor CT, Hagen T. Stabilization of hypoxia-inducible factor-1α protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J Biol Chem. 2010;285:31277–31284. doi: 10.1074/jbc.M110.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chun YS, Choi E, Kim TY, Kim MS, Park JW. A dominant-negative isoform lacking exons 11 and 12 of the human hypoxia-inducible factor-1α gene. Biochem J. 2002;362:71–79. doi: 10.1042/0264-6021:3620071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chun YS, Choi E, Yeo EJ, Lee JH, Kim MS, Park JW. A new HIF-1 α variant induced by zinc ion suppresses HIF-1-mediated hypoxic responses. J Cell Sci. 2001;114:4051–4061. doi: 10.1242/jcs.114.22.4051. [DOI] [PubMed] [Google Scholar]
- 52.Chun YS, Lee KH, Choi E, Bae SY, Yeo EJ, Huang LE, Kim MS, Park JW. Phorbol ester stimulates the nonhypoxic induction of a novel hypoxia-inducible factor 1α isoform: implications for tumor promotion. Cancer Res. 2003;63:8700–8707. [PubMed] [Google Scholar]
- 53.Cistulli P, Sullivan C. Dekker; New York: 1994. Pathophysiology of sleep apnea. In: Sleep and Breathing, edited by Saunders N, Sullivan C. pp. 405–448. [Google Scholar]
- 54.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen G, Roux JC, Grailhe R, Malcolm G, Changeux JP, Lagercrantz H. Perinatal exposure to nicotine causes deficits associated with a loss of nicotinic receptor function. Proc Natl Acad Sci USA. 2005;102:3817–3821. doi: 10.1073/pnas.0409782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Connolly MJ, Aaronson PI. Cell redox state and hypoxic pulmonary vasoconstriction: recent evidence and possible mechanisms. Respir Physiol Neurobiol. 2010;174:165–174. doi: 10.1016/j.resp.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Costa-Giomi P, Caro J, Weinmann R. Enhancement by hypoxia of human erythropoietin gene transcription in vitro. J Biol Chem. 1990;265:10185–10188. [PubMed] [Google Scholar]
- 58.Crosby ME, Devlin CM, Glazer PM, Calin GA, Ivan M. Emerging roles of microRNAs in the molecular responses to hypoxia. Curr Pharm Des. 2009;15:3861–3866. doi: 10.2174/138161209789649367. [DOI] [PubMed] [Google Scholar]
- 59.D'Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- 60.Dann CE, 3rd, Bruick RK, Deisenhofer J. Structure of factor-inhibiting hypoxia-inducible factor 1: an asparaginyl hydroxylase involved in the hypoxic response pathway. Proc Natl Acad Sci USA. 2002;99:15351–15356. doi: 10.1073/pnas.202614999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, Mazure NM. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1α. Cancer Res. 2006;66:3688–3698. doi: 10.1158/0008-5472.CAN-05-4564. [DOI] [PubMed] [Google Scholar]
- 62.Del Toro R, Levitsky KL, Lopez-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem. 2003;278:22316–22324. doi: 10.1074/jbc.M212576200. [DOI] [PubMed] [Google Scholar]
- 63.Dempsey JA, Forster HV. Mediation of ventilatory adaptations. Physiol Rev. 1982;62:262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- 64.Deng H, Hershenson MB, Lei J, Anyanwu AC, Pinsky DJ, Bentley JK. Pulmonary artery smooth muscle hypertrophy: roles of glycogen synthase kinase-3β and p70 ribosomal S6 kinase. Am J Physiol Lung Cell Mol Physiol. 2010;298:L793–L803. doi: 10.1152/ajplung.00108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhillon DP, Barer GR, Walsh M. The enlarged carotid body of the chronically hypoxic and chronically hypoxic and hypercapnic rat: a morphometric analysis. Q J Exp Physiol. 1984;69:301–317. doi: 10.1113/expphysiol.1984.sp002807. [DOI] [PubMed] [Google Scholar]
- 66.Di Giulio C, Bianchi G, Cacchio M, Artese L, Rapino C, Macri MA, Di Ilio C. Oxygen and life span: chronic hypoxia as a model for studying HIF-1α, VEGF and NOS during aging. Respir Physiol Neurobiol. 2005;147:31–38. doi: 10.1016/j.resp.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Di Giulio C, Grilli A, De Lutiis MA, Di Natale F, Sabatino G, Felaco M. Does chronic hypoxia increase rat carotid body nitric oxide? Comp Biochem Physiol A Mol Integr Physiol. 1998;120:243–247. doi: 10.1016/s1095-6433(98)00023-3. [DOI] [PubMed] [Google Scholar]
- 68.Diebold I, Petry A, Djordjevic T, Belaiba RS, Fineman J, Black S, Schreiber C, Fratz S, Hess J, Kietzmann T, Gorlach A. Reciprocal regulation of Rac1 and PAK-1 by HIF-1α: a positive-feedback loop promoting pulmonary vascular remodeling. Antioxid Redox Signal. 2010;13:399–412. doi: 10.1089/ars.2009.3013. [DOI] [PubMed] [Google Scholar]
- 69.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 70.Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA. HIF-2α-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest Ophthalmol Vis Sci. 2008;49:2714–2720. doi: 10.1167/iovs.07-1469. [DOI] [PubMed] [Google Scholar]
- 71.Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L318–L325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- 72.Discher DJ, Bishopric NH, Wu X, Peterson CA, Webster KA. Hypoxia regulates beta-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J Biol Chem. 1998;273:26087–26093. doi: 10.1074/jbc.273.40.26087. [DOI] [PubMed] [Google Scholar]
- 73.Doege K, Heine S, Jensen I, Jelkmann W, Metzen E. Inhibition of mitochondrial respiration elevates oxygen concentration but leaves regulation of hypoxia-inducible factor (HIF) intact. Blood. 2005;106:2311–2317. doi: 10.1182/blood-2005-03-1138. [DOI] [PubMed] [Google Scholar]
- 74.Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J. Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-independent mechanisms. J Biol Chem. 2001;276:18702–18709. doi: 10.1074/jbc.M011774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eckardt KU, Ratcliffe PJ, Tan CC, Bauer C, Kurtz A. Age-dependent expression of the erythropoietin gene in rat liver and kidneys. J Clin Invest. 1992;89:753–760. doi: 10.1172/JCI115652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 77.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 78.Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J Biol Chem. 2006;281:15215– 15226. doi: 10.1074/jbc.M511408200. [DOI] [PubMed] [Google Scholar]
- 79.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLHPAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 81.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 82.Evinger MJ, Cikos S, Nwafor-Anene V, Powers JF, Tischler AS. Hypoxia activates multiple transcriptional pathways in mouse pheochromocytoma cells. Ann NY Acad Sci. 2002;971:61–65. doi: 10.1111/j.1749-6632.2002.tb04434.x. [DOI] [PubMed] [Google Scholar]
- 83.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 84.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor 1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Filippi S, Latini P, Frontini M, Palitti F, Egly JM, Proietti-De-Santis L. CSB protein is (a direct target of HIF-1 and) a critical mediator of the hypoxic response. EMBO J. 2008;27:2545–2556. doi: 10.1038/emboj.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- 87.Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxiainducible factor-1α and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 88.Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 89.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1059–L1072. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 93.Fukuda R, Kelly B, Semenza GL. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330–2334. [PubMed] [Google Scholar]
- 94.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 95.Fung ML. Hypoxia-inducible factor-1: a molecular hint of physiological changes in the carotid body during long-term hypoxemia? Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:254–259. doi: 10.2174/1568006033481447. [DOI] [PubMed] [Google Scholar]
- 96.Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 97.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33:1195–1205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 98.Glasgow JN, Qiu J, Rassin D, Grafe M, Wood T, Perez-Pol JR. Transcriptional regulation of the BCL-X gene by NF-kappaB is an element of hypoxic responses in the rat brain. Neurochem Res. 2001;26:647–659. doi: 10.1023/a:1010987220034. [DOI] [PubMed] [Google Scholar]
- 99.Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- 100.Goldberg MA, Gaut CC, Bunn HF. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991;77:271–277. [PubMed] [Google Scholar]
- 101.Goldberg MA, Glass GA, Cunningham JM, Bunn HF. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci USA. 1987;84:7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gothie E, Richard DE, Berra E, Pages G, Pouyssegur J. Identification of alternative spliced variants of human hypoxia-inducible factor-1α. J Biol Chem. 2000;275:6922–6927. doi: 10.1074/jbc.275.10.6922. [DOI] [PubMed] [Google Scholar]
- 103.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 104.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2α results in anemia. Proc Natl Acad Sci USA. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Lands-man L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 106.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF-1α. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 109.Hampton-Smith RJ, Peet DJ. From polyps to people: a highly familiar response to hypoxia. Ann NY Acad Sci. 2009;1177:19–29. doi: 10.1111/j.1749-6632.2009.05035.x. [DOI] [PubMed] [Google Scholar]
- 110.Hartsfield CL, Alam J, Choi AM. Differential signaling pathways of HO-1 gene expression in pulmonary and systemic vascular cells. Am J Physiol Lung Cell Mol Physiol. 1999;277:L1133–L1141. doi: 10.1152/ajplung.1999.277.6.L1133. [DOI] [PubMed] [Google Scholar]
- 111.Heath D, Smith P, Fitch R, Harris P. Comparative pathology of the enlarged carotid body. J Comp Pathol. 1985;95:259–271. doi: 10.1016/0021-9975(85)90012-x. [DOI] [PubMed] [Google Scholar]
- 112.Hehlgans T, Seitz C, Lewis C, Mannel DN. Hypoxic upregulation of TNF receptor type 2 expression involves NF-IL-6 and is independent of HIF-1 or HIF-2. J Interferon Cytokine Res. 2001;21:757–762. doi: 10.1089/107999001753124480. [DOI] [PubMed] [Google Scholar]
- 113.Henze AT, Riedel J, Diem T, Wenner J, Flamme I, Pouyseggur J, Plate KH, Acker T. Prolyl hydroxylases 2 and 3 act in gliomas as protective negative feedback regulators of hypoxia-inducible factors. Cancer Res. 2010;70:357–366. doi: 10.1158/0008-5472.CAN-09-1876. [DOI] [PubMed] [Google Scholar]
- 114.Hewitson KS, Lienard BM, McDonough MA, Clifton IJ, Butler D, Soares AS, Oldham NJ, McNeill LA, Schofield CJ. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 115.Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 116.Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 117.Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M, Perdew GH, Bradfield CA. Characterization of a subset of the basic-helix-loop-helix-PAS super-family that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 118.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Pahlman S. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 119.Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol. 2007;50:697–702. doi: 10.1097/FJC.0b013e3181593774. [DOI] [PubMed] [Google Scholar]
- 120.Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, Ellisen LW. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA. 2010;107:4675–4680. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1α and HIF-2α. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxiainducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 125.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 127.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ilangovan G, Zweier JL, Kuppusamy P. Microximetry: simultaneous determination of oxygen consumption and free radical production using electron paramagnetic resonance spectroscopy. Methods Enzymol. 2004;381:747–762. doi: 10.1016/S0076-6879(04)81048-6. [DOI] [PubMed] [Google Scholar]
- 129.Isaac DD, Andrew DJ. Tubulogenesis in Drosophila: a requirement for the trachealess gene product. Genes Dev. 1996;10:103–117. doi: 10.1101/gad.10.1.103. [DOI] [PubMed] [Google Scholar]
- 130.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 131.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 132.Isaacs JS, Jung YJ, Neckers L. Aryl hydrocarbon nuclear translocator (ARNT) promotes oxygen-independent stabilization of hypoxia-inducible factor-1α by modulating an Hsp90-dependent regulatory pathway. J Biol Chem. 2004;279:16128–16135. doi: 10.1074/jbc.M313342200. [DOI] [PubMed] [Google Scholar]
- 133.Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxiainducible factor. Proc Natl Acad Sci USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 135.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1α gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. doi: 10.1006/geno.1998.5416. [DOI] [PubMed] [Google Scholar]
- 137.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 138.Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 139.Jeong CH, Lee HJ, Cha JH, Kim JH, Kim KR, Yoon DK, Kim KW. Hypoxia-inducible factor-1 α inhibits self-renewal of mouse embryonic stem cells in vitro via negative regulation of the leukemia inhibitory factor-STAT3 pathway. J Biol Chem. 2007;282:13672–13679. doi: 10.1074/jbc.M700534200. [DOI] [PubMed] [Google Scholar]
- 140.Ji YS, Xu Q, Schmedtje JF., Jr Hypoxia induces high-mobility-group protein I(Y) and transcription of the cyclooxygenase-2 gene in human vascular endothelium. Circ Res. 1998;83:295–304. doi: 10.1161/01.res.83.3.295. [DOI] [PubMed] [Google Scholar]
- 141.Jiang BH, Agani F, Passaniti A, Semenza GL. V-SRC induces expression of hypoxiainducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328–5335. [PubMed] [Google Scholar]
- 142.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 143.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol Cell Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 144.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1α. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 145.Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jyung RW, LeClair EE, Bernat RA, Kang TS, Ung F, McKenna MJ, Tuan RS. Expression of angiogenic growth factors in paragangliomas. Laryngoscope. 2000;110:161–167. doi: 10.1097/00005537-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 148.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 149.Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxiainducible transcription factor 1α by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 150.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci USA. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 152.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 153.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 154.Khanna S, Roy S, Maurer M, Ratan RR, Sen CK. Oxygen-sensitive reset of hypoxiainducible factor transactivation response: prolyl hydroxylases tune the biological normoxic set point. Free Radic Biol Med. 2006;40:2147–2154. doi: 10.1016/j.freeradbiomed.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 156.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 157.Kim SH, Xia D, Kim SW, Holla V, Menter DG, Dubois RN. Human enhancer of filamentation 1 is a mediator of hypoxia-inducible factor-1α-mediated migration in colorectal carcinoma cells. Cancer Res. 2010;70:4054–4063. doi: 10.1158/0008-5472.CAN-09-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D'Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- 159.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. Proc Natl Acad Sci USA. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koh MY, Lemos R, Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- 162.Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282:4524–4532. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 163.Kong T, Scully M, Shelley CS, Colgan SP. Identification of Pur α as a new hypoxia response factor responsible for coordinated induction of the beta 2 integrin family. J Immunol. 2007;179:1934–1941. doi: 10.4049/jimmunol.179.3.1934. [DOI] [PubMed] [Google Scholar]
- 164.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. HIF-1α induces genetic instability by transcriptionally downregulating MutSα expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 166.Koulmann N, Novel-Chate V, Peinnequin A, Chapot R, Serrurier B, Simler N, Richard H, Ventura-Clapier R, Bigard X. Cyclosporin A inhibits hypoxia-induced pulmonary hypertension and right ventricle hypertrophy. Am J Respir Crit Care Med. 2006;174:699–705. doi: 10.1164/rccm.200512-1976OC. [DOI] [PubMed] [Google Scholar]
- 167.Koury ST, Bondurant MC, Koury MJ, Semenza GL. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991;77:2497–2503. [PubMed] [Google Scholar]
- 168.Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–651. [PubMed] [Google Scholar]
- 169.Krick S, Hanze J, Eul B, Savai R, Seay U, Grimminger F, Lohmeyer J, Klepetko W, Seeger W, Rose F. Hypoxia-driven proliferation of human pulmonary artery fibro-blasts: cross-talk between HIF-1α and an autocrine angiotensin system. FASEB J. 2005;19:857–859. doi: 10.1096/fj.04-2890fje. [DOI] [PubMed] [Google Scholar]
- 170.Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1α enhances hypoxic gene expression and tumor growth. Mol Cell Biol. 2010;30:344–353. doi: 10.1128/MCB.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kumar P, Prabhakar N. Peripheral chemoreceptors: function and plasticity of the carotid body. Comprehensive Physiology. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kuwahara F, Kai H, Tokuda K, Shibata R, Kusaba K, Tahara N, Niiyama H, Nagata T, Imaizumi T. Hypoxia-inducible factor-1α/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension. 2002;39:46–50. doi: 10.1161/hy1201.097200. [DOI] [PubMed] [Google Scholar]
- 174.Kuwaki T, Cao WH, Kurihara Y, Kurihara H, Ling GY, Onodera M, Ju KH, Yazaki Y, Kumada M. Impaired ventilatory responses to hypoxia and hypercapnia in mutant mice deficient in endothelin-1. Am J Physiol Regul Integr Comp Physiol. 1996;270:R1279–R1286. doi: 10.1152/ajpregu.1996.270.6.R1279. [DOI] [PubMed] [Google Scholar]
- 175.Kwast KE, Burke PV, Poyton RO. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J Exp Biol. 1998;201:1177–1195. doi: 10.1242/jeb.201.8.1177. [DOI] [PubMed] [Google Scholar]
- 176.Lagercrantz H, Marcus C. Sympathoadrenal mechanism during development. In: Pollard R, Fox W, editors. Fetal and Neonatal Physiology. Saunders; Philadelphia, PA: 1992. [Google Scholar]
- 177.Laidler P, Kay JM. A quantitative study of some ultrastructural features of the type I cells in the carotid bodies of rats living at a simulated altitude of 4300 metres. J Neurocytol. 1978;7:183–192. doi: 10.1007/BF01217917. [DOI] [PubMed] [Google Scholar]
- 178.Lam SY, Leung PS. Chronic hypoxia activates a local angiotensin-generating system in rat carotid body. Mol Cell Endocrinol. 2003;203:147–153. doi: 10.1016/s0303-7207(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 179.Lam SY, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxiainducible factor-1α, -2α and -3α in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23:271–280. doi: 10.14670/HH-23.271. [DOI] [PubMed] [Google Scholar]
- 180.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxiainducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 182.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GC, Poon RT, Fan ST. An Akt/hypoxiainducible factor-1α/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res. 2009;15:3462–3471. doi: 10.1158/1078-0432.CCR-08-2127. [DOI] [PubMed] [Google Scholar]
- 184.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee C, Kim SJ, Jeong DG, Lee SM, Ryu SE. Structure of human FIH-1 reveals a unique active site pocket and interaction sites for HIF-1 and von Hippel-Lindau. J Biol Chem. 2003;278:7558–7563. doi: 10.1074/jbc.M210385200. [DOI] [PubMed] [Google Scholar]
- 186.Lee KH, Park JW, Chun YS. Non-hypoxic transcriptional activation of the aryl hydro-carbon receptor nuclear translocator in concert with a novel hypoxia-inducible factor-1α isoform. Nucleic Acids Res. 2004;32:5499–5511. doi: 10.1093/nar/gkh880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee KJ, Lee KY, Lee YM. Downregulation of a tumor suppressor RECK by hypoxia through recruitment of HDAC1 and HIF-1α to reverse HRE site in the promoter. Biochim Biophys Acta. 2010;1803:608–616. doi: 10.1016/j.bbamcr.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 188.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 189.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 190.Lei Z, Li B, Yang Z, Fang H, Zhang GM, Feng ZH, Huang B. Regulation of HIF-1α and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009;4:e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 192.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1α stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Physiol Genomics. 2006;25:450–457. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 194.Licht AH, Muller-Holtkamp F, Flamme I, Breier G. Inhibition of hypoxia-inducible factor activity in endothelial cells disrupts embryonic cardiovascular development. Blood. 2006;107:584–590. doi: 10.1182/blood-2005-07-3033. [DOI] [PubMed] [Google Scholar]
- 195.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 196.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 197.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1α and is required for O2-independent and HSP90 inhibitor-induced degradation of HIF-1α. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu YV, Hubbi ME, Pan F, McDonald KR, Mansharamani M, Cole RN, Liu JO, Semenza GL. Calcineurin promotes hypoxia-inducible factor 1α expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem. 2007;282:37064–37073. doi: 10.1074/jbc.M705015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lok CN, Ponka P. Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem. 1999;274:24147–24152. doi: 10.1074/jbc.274.34.24147. [DOI] [PubMed] [Google Scholar]
- 200.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 201.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 202.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 203.Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L, Wenger RH, Sitkovsky M. Cutting edge: hypoxia-inducible factor 1α and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol. 2006;177:4962–4965. doi: 10.4049/jimmunol.177.8.4962. [DOI] [PubMed] [Google Scholar]
- 204.Lukashev D, Sitkovsky M. Preferential expression of the novel alternative isoform I.3 of hypoxia-inducible factor 1α in activated human T lymphocytes. Hum Immunol. 2008;69:421–425. doi: 10.1016/j.humimm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 Is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luo W, Zhong J, Chang R, Hu H, Pandey A, Semenza GL. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but not HIF-2α. J Biol Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxiainducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 209.Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J Biol Chem. 2002;277:32405–32408. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- 210.Makino Y, Uenishi R, Okamoto K, Isoe T, Hosono O, Tanaka H, Kanopka A, Poellinger L, Haneda M, Morimoto C. Transcriptional up-regulation of inhibitory PAS domain protein gene expression by hypoxia-inducible factor 1 (HIF-1): a negative feedback regulatory circuit in HIF-1-mediated signaling in hypoxic cells. J Biol Chem. 2007;282:14073–14082. doi: 10.1074/jbc.M700732200. [DOI] [PubMed] [Google Scholar]
- 211.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 212.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 214.Marti HH, Katschinski DM, Wagner KF, Schaffer L, Stier B, Wenger RH. Isoformspecific expression of hypoxia-inducible factor-1α during the late stages of mouse spermiogenesis. Mol Endocrinol. 2002;16:234–243. doi: 10.1210/mend.16.2.0786. [DOI] [PubMed] [Google Scholar]
- 215.Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, Hohimer AR, Semenza GL. Cardiac hypertrophy in chronically anemic fetal sheep: increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol. 1998;178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 216.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Matsui H, Ihara Y, Fujio Y, Kunisada K, Akira S, Kishimoto T, Yamauchi-Takihara K. Induction of interleukin (IL)-6 by hypoxia is mediated by nuclear factor (NF)-kappa B and NF-IL6 in cardiac myocytes. Cardiovasc Res. 1999;42:104–112. doi: 10.1016/s0008-6363(98)00285-5. [DOI] [PubMed] [Google Scholar]
- 219.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 220.Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, Conaway RC, Conaway JW, Ohh M. Multiple splice variants of the human HIF-3α locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem. 2003;278:11032–11040. doi: 10.1074/jbc.M208681200. [DOI] [PubMed] [Google Scholar]
- 221.Mazure NM, Chauvet C, Bois-Joyeux B, Bernard MA, Nacer-Cherif H, Danan JL. Repression of α-fetoprotein gene expression under hypoxic conditions in human hepatoma cells: characterization of a negative hypoxia response element that mediates opposite effects of hypoxia inducible factor-1 and c-Myc. Cancer Res. 2002;62:1158–1165. [PubMed] [Google Scholar]
- 222.Mazza E, Thakkar-Varia S, Tozzi CA, Neubauer JA. Expression of heme oxygenase in the oxygen-sensing regions of the rostral ventrolateral medulla. J Appl Physiol. 2001;91:379–385. doi: 10.1152/jappl.2001.91.1.379. [DOI] [PubMed] [Google Scholar]
- 223.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S, Califano JA, Jeoung NH, Harris RA, Verma A. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McNeill LA, Hewitson KS, Claridge TD, Seibel JF, Horsfall LE, Schofield CJ. Hypoxiainducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the β-carbon of asparagine-803. Biochem J. 2002;367:571–575. doi: 10.1042/BJ20021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.McNeill LA, Hewitson KS, Gleadle JM, Horsfall LE, Oldham NJ, Maxwell PH, Pugh CW, Ratcliffe PJ, Schofield CJ. The use of dioxygen by HIF prolyl hydroxylase (PHD1). Bioorg Med Chem Lett. 2002;12:1547–1550. doi: 10.1016/s0960-894x(02)00219-6. [DOI] [PubMed] [Google Scholar]
- 226.Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, Varesio L. Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem. 1997;272:12236–12243. doi: 10.1074/jbc.272.18.12236. [DOI] [PubMed] [Google Scholar]
- 227.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 229.Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 1992;263:R368–R375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- 230.Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1α-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- 231.Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004;64:1765–1772. doi: 10.1158/0008-5472.can-03-3017. [DOI] [PubMed] [Google Scholar]
- 232.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Morio Y, McMurtry IF. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- 234.Morita M, Ohneda O, Yamashita T, Takahashi S, Suzuki N, Nakajima O, Kawauchi S, Ema M, Shibahara S, Udono T, Tomita K, Tamai M, Sogawa K, Yamamoto M, Fujii-Kuriyama Y. HLF/HIF-2α is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Morrell NWAS, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlewaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 54:2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mujcic H, Rzymski T, Rouschop KM, Koritzinsky M, Milani M, Harris AL, Wouters BG. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol. 2009;92:450–459. doi: 10.1016/j.radonc.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 237.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 238.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 240.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxi-some proliferator-activated receptor α expression during hypoxia. J Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 241.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 242.Nishi H, Nishi KH, Johnson AC. Early Growth Response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res. 2002;62:827–834. [PubMed] [Google Scholar]
- 243.Norris ML, Millhorn DE. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 244.O'Reilly SM, Leonard MO, Kieran N, Comerford KM, Cummins E, Pouliot M, Lee SB, Taylor CT. Hypoxia induces epithelial amphiregulin gene expression in a CREB-dependent manner. Am J Physiol Cell Physiol. 2006;290:C592–C600. doi: 10.1152/ajpcell.00278.2005. [DOI] [PubMed] [Google Scholar]
- 245.Oikawa M, Abe M, Kurosawa H, Hida W, Shirato K, Sato Y. Hypoxia induces transcription factor ETS-1 via the activity of hypoxia-inducible factor-1. Biochem Biophys Res Commun. 2001;289:39–43. doi: 10.1006/bbrc.2001.5927. [DOI] [PubMed] [Google Scholar]
- 246.Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M, Semenza GL. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- 247.Oparil S, Chen SJ, Meng QC, Elton TS, Yano M, Chen YF. Endothelin-A receptor antagonist prevents acute hypoxia-induced pulmonary hypertension in the rat. Am J Physiol Lung Cell Mol Physiol. 1995;268:L95–L100. doi: 10.1152/ajplung.1995.268.1.L95. [DOI] [PubMed] [Google Scholar]
- 248.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58:1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 249.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 250.Papkovsky DB. Methods in optical oxygen sensing: protocols and critical analyses. Methods Enzymol. 2004;381:715–735. doi: 10.1016/S0076-6879(04)81046-2. [DOI] [PubMed] [Google Scholar]
- 251.Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1α (HIF-1α): role of cytoplasmic trapping of HIF-2α. Mol Cell Biol. 2003;23:4959–4971. doi: 10.1128/MCB.23.14.4959-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pascual O, Denavit-Saubie M, Dumas S, Kietzmann T, Ghilini G, Mallet J, Pequignot JM. Selective cardiorespiratory and catecholaminergic areas express the hypoxiainducible factor-1α (HIF-1α) under in vivo hypoxia in rat brainstem. Eur J Neurosci. 2001;14:1981–1991. doi: 10.1046/j.0953-816x.2001.01816.x. [DOI] [PubMed] [Google Scholar]
- 253.Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2α (HIF-2α) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA. 2011;108:3065–3070. doi: 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 257.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pequignot JM, Hellstrom S, Johansson C. Intact and sympathectomized carotid bodies of long-term hypoxic rats: a morphometric ultrastructural study. J Neurocytol. 1984;13:481–493. doi: 10.1007/BF01148336. [DOI] [PubMed] [Google Scholar]
- 259.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 262.Powell FL, Fu Z. HIF-1 and ventilatory acclimatization to chronic hypoxia. Respir Physiol Neurobiol. 2008;164:282–287. doi: 10.1016/j.resp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 264.Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 265.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- 266.Prabhakar NR, Jacono FJ. Cellular and molecular mechanisms associated with carotid body adaptations to chronic hypoxia. High Alt Med Biol. 2005;6:112–120. doi: 10.1089/ham.2005.6.112. [DOI] [PubMed] [Google Scholar]
- 267.Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol. 2004;96:359–366. doi: 10.1152/japplphysiol.00809.2003. [DOI] [PubMed] [Google Scholar]
- 268.Prabhakar NR, Pieramici SF, Premkumar DR, Kumar GK, Kalaria RN. Activation of nitric oxide synthase gene expression by hypoxia in central and peripheral neurons. Brain Res. 1996;43:341–346. doi: 10.1016/s0169-328x(96)00222-7. [DOI] [PubMed] [Google Scholar]
- 269.Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. Activation of hypoxiainducible factor-1: definition of regulatory domains within the α subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 270.Pugh CW, Tan CC, Jones RW, Ratcliffe PJ. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3= to the mouse erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:10553–10557. doi: 10.1073/pnas.88.23.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Quintero M, Brennan PA, Thomas GJ, Moncada S. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1α in cancer: role of free radical formation. Cancer Res. 2006;66:770–774. doi: 10.1158/0008-5472.CAN-05-0333. [DOI] [PubMed] [Google Scholar]
- 273.Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol. 2001;90:2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- 277.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rey S, Lee K, Wang CJ, Gupta K, Chen S, McMillan A, Bhise N, Levchenko A, Semenza GL. Synergistic effect of HIF-1α gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci USA. 2009;106:20399–20404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Roeggla G, Roeggla M, Wagner A, Laggner AN. Poor ventilatory response to mild hypoxia may inhibit acclimatization at moderate altitude in elderly patients after carotid surgery. Br J Sports Med. 1995;29:110–112. doi: 10.1136/bjsm.29.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055–20062. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 282.Rossignol F, Vache C, Clottes E. Natural antisense transcripts of hypoxia-inducible factor 1α are detected in different normal and tumour human tissues. Gene. 2002;299:135–140. doi: 10.1016/s0378-1119(02)01049-1. [DOI] [PubMed] [Google Scholar]
- 283.Roux JC, Brismar H, Aperia A, Lagercrantz H. Developmental changes in HIF transcription factor in carotid body: relevance for O2 sensing by chemoreceptors. Pediatr Res. 2005;58:53–57. doi: 10.1203/01.PDR.0000163390.78239.EA. [DOI] [PubMed] [Google Scholar]
- 284.Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M, Strauch A, Orosz CG, Sen CK. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res. 2003;92:264–271. doi: 10.1161/01.res.0000056770.30922.e6. [DOI] [PubMed] [Google Scholar]
- 285.Salceda S, Caro J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 286.Sandau KB, Faus HG, Brune B. Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI3K pathway. Biochem Biophys Res Commun. 2000;278:263–267. doi: 10.1006/bbrc.2000.3789. [DOI] [PubMed] [Google Scholar]
- 287.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 288.Sato M, Severinghaus JW, Powell FL, Xu FD, Spellman MJ., Jr Augmented hypoxic ventilatory response in men at altitude. J Appl Physiol. 1992;73:101–107. doi: 10.1152/jappl.1992.73.1.101. [DOI] [PubMed] [Google Scholar]
- 289.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 290.Schuster SJ, Badiavas EV, Costa-Giomi P, Weinmann R, Erslev AJ, Caro J. Stimulation of erythropoietin gene transcription during hypoxia and cobalt exposure. Blood. 1989;73:13–16. [PubMed] [Google Scholar]
- 291.Schuster SJ, Wilson JH, Erslev AJ, Caro J. Physiologic regulation and tissue localization of renal erythropoietin messenger RNA. Blood. 1987;70:316–318. [PubMed] [Google Scholar]
- 292.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 293.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2α is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 294.Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells. Mol Cell Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol. 1985;358:1–16. doi: 10.1113/jphysiol.1985.sp015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 297.Semenza GL, Dureza RC, Traystman MD, Gearhart JD, Antonarakis SE. Human erythropoietin gene expression in transgenic mice: multiple transcription initiation sites and cis-acting regulatory elements. Mol Cell Biol. 1990;10:930–938. doi: 10.1128/mcb.10.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 299.Semenza GL, Koury ST, Nejfelt MK, Gearhart JD, Antonarakis SE. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci USA. 1991;88:8725–8729. doi: 10.1073/pnas.88.19.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3 to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Semenza GL, Rue EA, Iyer NV, Pang MG, Kearns WG. Assignment of the hypoxiainducible factor 1α gene to a region of conserved synteny on mouse chromosome 12 and human chromosome 14q. Genomics. 1996;34:437–439. doi: 10.1006/geno.1996.0311. [DOI] [PubMed] [Google Scholar]
- 302.Semenza GL, Traystman MD, Gearhart JD, Antonarakis SE. Polycythemia in transgenic mice expressing the human erythropoietin gene. Proc Natl Acad Sci USA. 1989;86:2301–2305. doi: 10.1073/pnas.86.7.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 305.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 306.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 307.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;279:L884–L894. doi: 10.1152/ajplung.2000.279.5.L884. [DOI] [PubMed] [Google Scholar]
- 308.Shizukuda Y, Mallet RT, Lee SC, Downey HF. Hypoxic preconditioning of ischaemic canine myocardium. Cardiovasc Res. 1992;26:534–542. doi: 10.1093/cvr/26.5.534. [DOI] [PubMed] [Google Scholar]
- 309.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 310.Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 311.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2α (HIF-2α) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Res Bull. 1995;38:69–75. doi: 10.1016/0361-9230(95)00073-n. [DOI] [PubMed] [Google Scholar]
- 313.Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, Percy MJ, Pugh CW, Ratcliffe PJ, Talbot NP, Treacy M, Robbins PA. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3:e290. doi: 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Soliz J, Joseph V, Soulage C, Becskei C, Vogel J, Pequignot JM, Ogunshola O, Gassmann M. Erythropoietin regulates hypoxic ventilation in mice by interacting with brainstem and carotid bodies. J Physiol. 2005;568:559–571. doi: 10.1113/jphysiol.2005.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 316.Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxiainducible transcription factor (Hif)-1α versus Hif-2α in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- 319.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 320.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM. Regulation of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003;163:1001–1011. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999;274:24142–24146. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- 323.Tai TC, Wong-Faull DC, Claycomb R, Wong DL. Hypoxia and adrenergic function: molecular mechanisms related to Egr-1 and Sp1 activation. Brain Res. 2010;1353:14–27. doi: 10.1016/j.brainres.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 324.Tajima M, Katayose D, Bessho M, Isoyama S. Acute ischaemic preconditioning and chronic hypoxia independently increase myocardial tolerance to ischaemia. Cardiovasc Res. 1994;28:312–319. doi: 10.1093/cvr/28.3.312. [DOI] [PubMed] [Google Scholar]
- 325.Tan M, Gu Q, He H, Pamarthy D, Semenza GL, Sun Y. SAG/ROC2/RBX2 is a HIF-1 target gene that promotes HIF-1α ubiquitination and degradation. Oncogene. 2008;27:1404–1411. doi: 10.1038/sj.onc.1210780. [DOI] [PubMed] [Google Scholar]
- 326.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 327.Taylor CT, Fueki N, Agah A, Hershberg RM, Colgan SP. Critical role of cAMP response element binding protein expression in hypoxia-elicited induction of epithelial tumor necrosis factor-α. J Biol Chem. 1999;274:19447–19454. doi: 10.1074/jbc.274.27.19447. [DOI] [PubMed] [Google Scholar]
- 328.Thornton JD, Liu GS, Olsson RA, Downey JM. Intravenous pretreatment with A1-selective adenosine analogs protects the heart against infarction. Circulation. 1992;85:659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- 329.Thrash-Bingham CA, Tartof KD. aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst. 1999;91:143–151. doi: 10.1093/jnci/91.2.143. [DOI] [PubMed] [Google Scholar]
- 330.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 332.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxiainducible factors 1α and 2α on mTORC1 and mTORC2. J Biol Chem. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 334.Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 336.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1α and HIF-2α expression in lung epithelial cells: implication of natural antisense HIF-1α. J Biol Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 337.Van den Beucken T, Koritzinsky M, Niessen H, Dubois L, Savelkouls K, Mujcic H, Jutten B, Kopacek J, Pastorekova S, van der Kogel AJ, Lambin P, Voncken W, Rouschop KM, Wouters BG. Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. J Biol Chem. 2009;284:24204–24212. doi: 10.1074/jbc.M109.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Van Royen N, Hoefer I, Bottinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92:218–225. doi: 10.1161/01.res.0000052313.23087.3f. [DOI] [PubMed] [Google Scholar]
- 339.Varia MA, Calkins-Adams DP, Rinker LH, Kennedy AS, Novotny DB, Fowler WC, Jr, Raleigh JA. Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol Oncol. 1998;71:270–277. doi: 10.1006/gyno.1998.5163. [DOI] [PubMed] [Google Scholar]
- 340.Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol. 1987;63:1658–1664. doi: 10.1152/jappl.1987.63.4.1658. [DOI] [PubMed] [Google Scholar]
- 341.Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol. 1987;63:2403–2410. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]
- 342.Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–292. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 343.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 345.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 346.Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC, Marsden PA. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest. 2005;115:3128–3139. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2α target gene in Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 348.Wenger RH, Rolfs A, Marti HH, Guenet JL, Gassmann M. Nucleotide sequence, chromosomal assignment and mRNA expression of mouse hypoxia-inducible factor-1α. Biochem Biophys Res Commun. 1996;223:54–59. doi: 10.1006/bbrc.1996.0845. [DOI] [PubMed] [Google Scholar]
- 349.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 350.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2008;294:L309–L318. doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]
- 351.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxiainducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 352.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 353.Wilk R, Weizman I, Shilo BZ. trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes Dev. 1996;10:93–102. doi: 10.1101/gad.10.1.93. [DOI] [PubMed] [Google Scholar]
- 354.Wilson DF. Identifying oxygen sensors by their photochemical action spectra. Methods Enzymol. 2004;381:690–704. doi: 10.1016/S0076-6879(04)81044-9. [DOI] [PubMed] [Google Scholar]
- 355.Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Siddall BJ, Bell RA, Kvetnansky R. Stress and adrenergic function: HIF1α, a potential regulatory switch. Cell Mol Neurobiol. 2010;30:1451–1457. doi: 10.1007/s10571-010-9567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, Gerasimovskaya EV. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L954–L964. doi: 10.1152/ajplung.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci USA. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Xu L, Xie K, Mukaida N, Matsushima K, Fidler IJ. Hypoxia-induced elevation in inter-leukin-8 expression by human ovarian carcinoma cells. Cancer Res. 1999;59:5822–5829. [PubMed] [Google Scholar]
- 359.Xu Q, Ji YS, Schmedtje JF., Jr Sp1 increases expression of cyclooxygenase-2 in hypoxic vascular endothelium. Implications for the mechanisms of aortic aneurysm and heart failure. J Biol Chem. 2000;275:24583–24589. doi: 10.1074/jbc.M003894200. [DOI] [PubMed] [Google Scholar]
- 360.Xu Y, Zuo Y, Zhang H, Kang X, Yue F, Yi Z, Liu M, Yeh ET, Chen G, Cheng J. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J Biol Chem. 2010;285:36682–36688. doi: 10.1074/jbc.M110.164236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 362.Yan SF, Lu J, Zou YS, Kisiel W, Mackman N, Leitges M, Steinberg S, Pinsky D, Stern D. Protein kinase C-beta and oxygen deprivation. A novel Egr-1-dependent pathway for fibrin deposition in hypoxemic vasculature. J Biol Chem. 2000;275:11921–11928. doi: 10.1074/jbc.275.16.11921. [DOI] [PubMed] [Google Scholar]
- 363.Yan SF, Zou YS, Gao Y, Zhai C, Mackman N, Lee SL, Milbrandt J, Pinsky D, Kisiel W, Stern D. Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA. 1998;95:8298–8303. doi: 10.1073/pnas.95.14.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Yan SF, Zou YS, Mendelsohn M, Gao Y, Naka Y, Du Yan S, Pinsky D, Stern D. Nuclear factor interleukin 6 motifs mediate tissue-specific gene transcription in hypoxia. J Biol Chem. 1997;272:4287–4294. doi: 10.1074/jbc.272.7.4287. [DOI] [PubMed] [Google Scholar]
- 365.Yao G, Harstad E, Bradfield C. In: The Ah receptor. In: PAS Proteins: Regulators and Sensors of Development and Physiology. Crews ST, Norwell MA, editors. Kluwer Academic; 2003. pp. 149–182. [Google Scholar]
- 366.Ye JS, Tipoe GL, Fung PC, Fung ML. Augmentation of hypoxia-induced nitric oxide generation in the rat carotid body adapted to chronic hypoxia: an involvement of constitutive and inducible nitric oxide synthases. Pflügers Arch. 2002;444:178–185. doi: 10.1007/s00424-002-0785-1. [DOI] [PubMed] [Google Scholar]
- 367.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Yang H, Nielsen R, Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 369.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol Lung Cell Mol Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 370.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Yu F, White SB, Zhao Q, Lee FS. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226:2925–2933. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 374.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- 376.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 379.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F, Johnson RS. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 2010;11:364–378. doi: 10.1016/j.cmet.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zhang R, Wu Y, Zhao M, Liu C, Zhou L, Shen S, Liao S, Yang K, Li Q, Wan H. Role of HIF-1α in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 382.Zhao P, Zhao Y, Sherry AD, Pantano P. Concurrent 31P nuclear magnetic resonance spectroscopy and fiber-optic oxygen consumption measurements in perfused rat hearts. Methods Enzymol. 2004;381:735–746. doi: 10.1016/S0076-6879(04)81047-4. [DOI] [PubMed] [Google Scholar]
- 383.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]