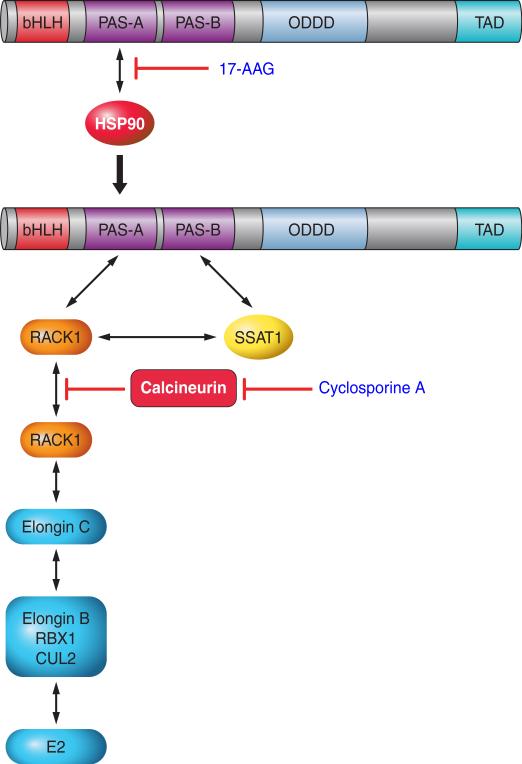
FIGURE 5.
Ubiquitination of HIF-1α by RACK1. Inhibitors of HSP90, such as 17-allylamino-17-demethoxygeldanamycin (17-AAG), block the binding of HSP90 to the PAS-A domain of HIF-1α, allowing RACK1 to bind at this site and recruit the ElonginC ubiquitin ligase complex. Calcineurin dephosphorylates RACK1, thereby preventing its dimerization, which is necessary to bring the ElonginC complex into contact with HIF-1α. Calcineurin activity is inhibited by cyclosporine A, which thereby increases ubiquitination of HIF-1α in a RACK1-dependent manner.