Figure 1.
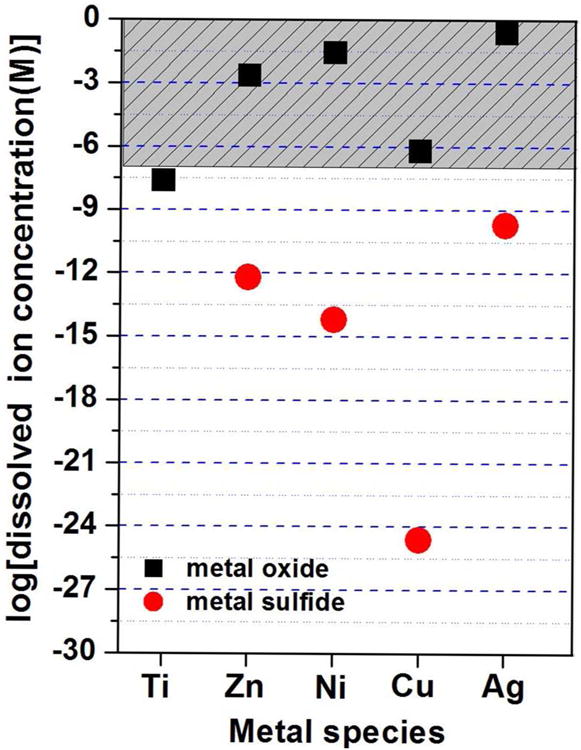
Equilibrium solubility of the oxide and sulfide forms of common metal-based nanoparticles. The equilibrium dissolved metal ion concentration was estimated using Visual MINTEQ 3.0 using bulk thermodynamic parameters.(See Supporting Information for details) The phases considered were TiO2, ZnO, NiO, CuO and Ag2O and the sulfides ZnS, NiS, CuS and Ag2S. The incubation conditions for the metal oxide calculations were aqueous solution (pH 7) and 1 mM NaNO3 as electrolyte, and for metal sulfide ligand-free aqueous solution (pH 7) containing an environmentally relevant total sulfide concentration (1 mM). Please note the calculations ignore any oxidative dissolution. Toxicity studies on free ions typically show adverse biological effects at micromolar doses (> 0.1 uM),59-62 which defines the shaded region above. Data points that fall in the shaded region all correspond to cases where nanoparticle toxicity has been attributed to dissolution mechanisms (oxides of Zn, Ni, Cu, Ag), which is possible due to the finite solubility of the oxide phase. TiO2 solubility in contrast is too low to yield ions above the micromolar range, where ion-induced toxicity is often seen in other systems.
